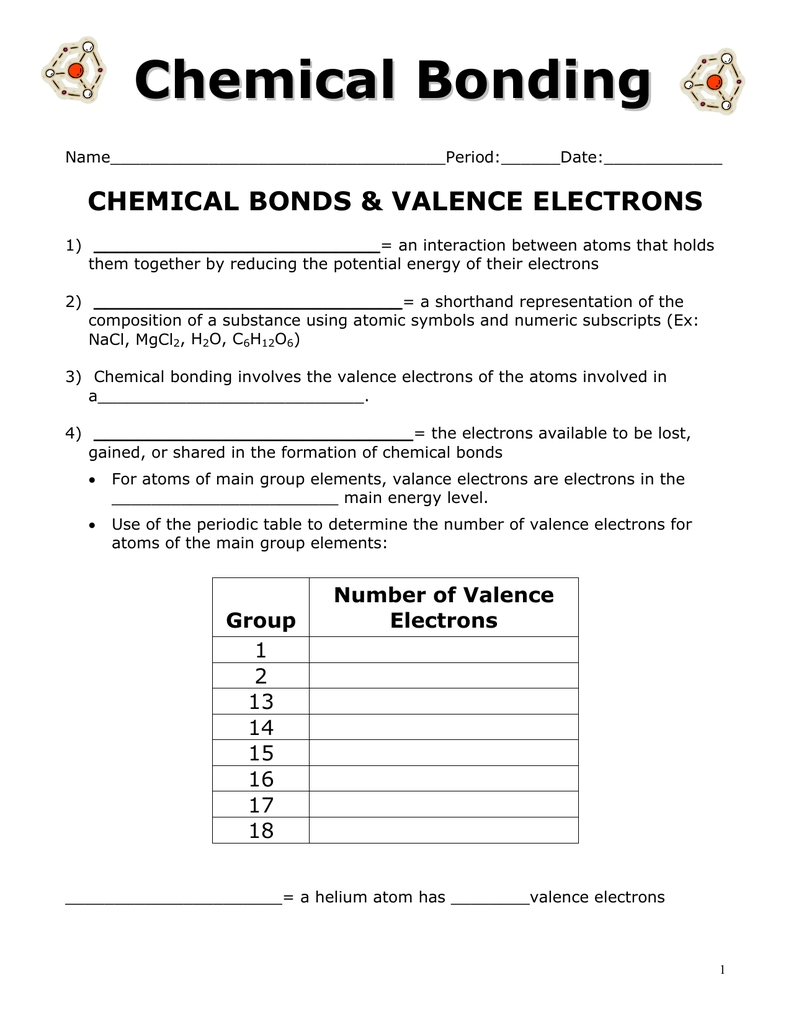
Exploring Valence Electrons in Chemistry: A Comprehensive Guide to Using a Valence Electrons Worksheet and Answers
Valence electrons play a critical role in chemistry, and understanding them is essential for accurately predicting the behavior of various elements. A valence electrons worksheet is a useful tool for helping students understand the relationship between the number of valence electrons and an element’s chemical properties. This comprehensive guide provides an overview of using a valence electrons worksheet, including how to read and interpret the results, as well as a set of sample questions and answers.
The valence electrons worksheet consists of two columns: one for the element and one for its valence electrons. Each element is listed with its atomic number, which is the number of protons in the nucleus of the atom. The valence electrons column shows the number of electrons in the outermost energy level of the atom. This is important because the number of valence electrons determines the element’s reactivity, as well as its ability to form bonds with other elements.
To use the valence electrons worksheet, begin by looking up the atomic number of the element you are interested in. Next, look in the valence electrons column to find the number of valence electrons for the element. This number should match the number of electrons in the outermost energy level of the atom. Finally, use this number to determine the element’s chemical behavior.
[toc]
For example, a carbon atom has six valence electrons, meaning it will form four covalent bonds with other elements. On the other hand, a chlorine atom has seven valence electrons, so it can form three covalent bonds with other elements. This can be used to predict the behavior of elements in chemical reactions.
To better understand how to use a valence electrons worksheet, it’s helpful to look at some examples. Consider the following questions and answers:
Q: How many valence electrons does a carbon atom have?
A: A carbon atom has six valence electrons.
Q: How many covalent bonds can a chlorine atom form?
A: A chlorine atom can form three covalent bonds.
Q: What is the reactivity of a sulfur atom?
A: A sulfur atom has six valence electrons, so it will be highly reactive and form multiple bonds with other elements.
By understanding how to read and interpret a valence electrons worksheet, students can gain a better understanding of the relationship between an element’s number of valence electrons and its chemical behavior. With this knowledge, they can better predict the outcome of chemical reactions and use the information to design experiments.
Examining Valence Electrons: How to Utilize a Valence Electrons Worksheet to Gain a Better Understanding of Molecular Structure
Valence electrons are an integral part of understanding molecular structure. Valence electrons are the outermost electrons of an atom and are responsible for most of the chemical behavior of the element. To gain a better understanding of how molecules are formed and interact, it is important to understand the valence electrons of each atom. A valence electrons worksheet can be a helpful tool in this regard.
The worksheet begins by listing the elements and their number of valence electrons. This information is essential to understanding the molecular structure of the molecule being studied. For each element, the worksheet provides a diagram of the atom’s valence electrons. This diagram is important as it shows the electrons in the outermost shell of the atom and how they interact with each other. It also provides a visual representation of the electron distribution in the atom.
Next, the worksheet provides a section for determining the number of covalent bonds that are formed between the atoms. Covalent bonds are formed when two atoms share electrons. These bonds are responsible for much of the stability of molecules. The worksheet also provides a section for calculating the total number of valence electrons in the molecule. This is important for predicting the overall stability and reactivity of the molecule.
Finally, the worksheet provides a section for connecting the valence electrons of the atoms. This section is important for understanding how electrons interact with each other in the molecule and how this affects the overall stability and reactivity of the molecule.
By utilizing a valence electrons worksheet, individuals can gain an improved understanding of how molecules are formed and interact. The worksheet provides a visual representation of the electron distribution and an easy way to calculate the number of covalent bonds between atoms. Additionally, it provides a way to connect the valence electrons of each atom to gain an understanding of how they interact with each other in the molecule. Understanding these concepts is essential to gaining a better understanding of molecular structure and predicting the reactivity of molecules.
Bonding Basics: A Step-by-Step Guide to Using a Valence Electrons Worksheet and Answers to Calculate Bond Strengths
Step 1: Become Familiar with Valence Electrons
Before beginning any calculations, it is important to understand what valence electrons are and why they are important. Valence electrons are the outermost electrons of an atom and are involved in the formation of chemical bonds. The number of valence electrons an atom has is determined by its position in the periodic table. For example, helium has two valence electrons, oxygen has six, and carbon has four.
Step 2: Gather Necessary Materials
To calculate the bond strength of a chemical bond, you will need a valence electrons worksheet and a calculator. The worksheet should include information about the atoms involved in the bond, such as the number of valence electrons and the electronegativity of each atom.
Step 3: Calculate the Bond Strength
Using the worksheet, enter the number of valence electrons of each atom in the formula and calculate the bond strength. The formula for calculating bond strength is: Bond Strength = (Number of Valence Electrons) + (Electronegativity of both atoms). For example, if an atom has four valence electrons and its partner atom has two valence electrons and an electronegativity of 3.3, the bond strength would be calculated as follows: Bond Strength = (4 + 2) + (3.3 + 3.3) = 12.6.
Step 4: Answer Questions and Interpret Results
After calculating the bond strength, answer any questions related to the bond strength and interpret the results. For example, if the bond strength is greater than 12, it indicates a strong bond. If it is lower than 12, it indicates a weak bond.
Step 5: Repeat Process for Other Bonds
After completing the process for one chemical bond, repeat the steps for any other bonds that need to be calculated. The results should provide a better understanding of the bond strengths between different atoms and molecules.
Conclusion
The Valence Electrons Worksheet Answers provide a great opportunity for students to explore the basics of chemistry and to gain a deeper understanding of the structure of atoms. By completing the worksheet, students have a better understanding of the importance of valence electrons and their role in chemical reactions. With this knowledge, they can make informed decisions when choosing materials and reactants to use in their experiments.
[addtoany]