Exploring Quantum Numbers: Understanding the Basics with a Quantum Numbers Worksheet
Quantum numbers are an important concept in the world of quantum mechanics. Understanding quantum numbers is key to understanding the structure of atoms and molecules and the behavior of electrons. To help students gain an understanding of quantum numbers, a quantum numbers worksheet can be used.
The quantum numbers worksheet should include an overview of the four quantum numbers (principal, angular momentum, magnetic, and spin) and their associated values. It should also provide an explanation of the meaning of each quantum number and how they interact with each other.
The worksheet should also include a number of exercises. These exercises should include questions designed to help students develop an understanding of the basic principles of quantum numbers. For example, the worksheet might include questions that ask students to identify the quantum numbers associated with a given electron configuration.
[toc]
The worksheet should also include an explanation of the Heisenberg uncertainty principle and how it relates to quantum numbers. This principle states that it is impossible to know the exact values of quantum numbers at the same time. This is an important concept to understand when studying quantum mechanics.
Finally, the worksheet should provide an opportunity for students to practice applying the principles of quantum numbers to real-world problems. For example, students may be asked to calculate the energy of a hydrogen atom given its quantum numbers. This will help them gain a better understanding of how quantum numbers can be used to predict the behavior of electrons.
By using a quantum numbers worksheet, students can gain a better understanding of the basics of quantum mechanics and the behavior of electrons. With this knowledge, they will be better prepared to tackle more advanced topics in quantum mechanics.
Demystifying the Quantum Numbers: A Comprehensive Guide to Quantum Numbers Worksheet Answers
Quantum numbers are an essential part of the quantum theory, which describes the behavior of particles such as atoms and electrons. The quantum numbers are mathematical expressions used to describe the states of these particles. They are used to explain the energy levels of particles, as well as the spatial orientation of particles. In this guide, we will discuss the various quantum numbers, their meanings, and how they are used in physics and chemistry.
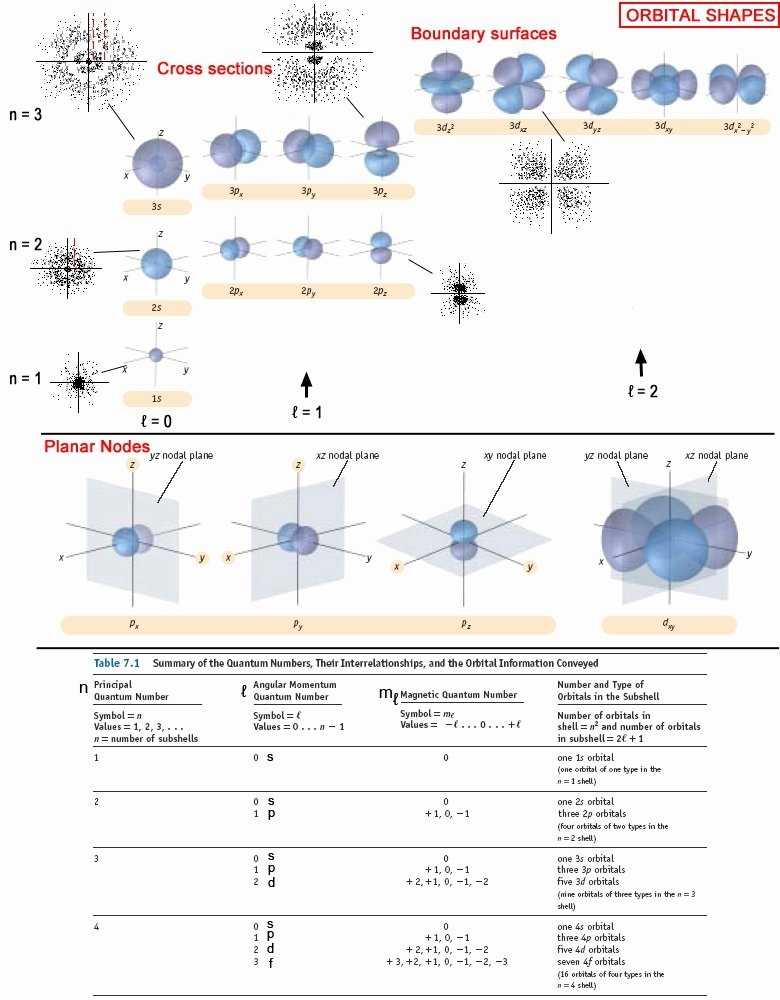
The four primary quantum numbers are n, l, mₑ, and ms. The principal quantum number, n, describes the energy state of a particle. It is an integer that can range from 1 to infinity. The angular momentum quantum number, l, describes the shape of the electron orbital. It is also an integer that can range from 0 to n-1. The magnetic quantum number, mₑ, describes the orientation of the orbital in space. It can range from -l to +l. The spin quantum number, ms, describes the spin of the electron. It can take on two values, +1/2 and -1/2.
These four quantum numbers can be used to calculate the energy states of a particle, which is important for understanding its behavior. For example, the energy states of an electron can be determined by calculating its four quantum numbers. The energy of an electron at a particular state is given by the equation E = -hₒ2/2m(n² + l + 1/2).
The four quantum numbers can also be used to explain the behavior of electrons in atoms. Electrons in an atom are arranged in shells, which are determined by the principal quantum number. The angular momentum quantum number describes the shape of the electron orbital, while the magnetic quantum number determines the orientation of the orbital. The spin quantum number describes the spin of the electron.
Quantum numbers are used in physics and chemistry to explain the behavior of particles, including electrons in atoms. By understanding the four primary quantum numbers, n, l, mₑ, and ms, we gain insight into the behavior of particles such as atoms and electrons. This knowledge is essential for understanding the behavior of the universe on the quantum level.
A Step-by-Step Guide to Answering Quantum Numbers Worksheet Questions
1. Identify the quantum numbers: Start by understanding what quantum numbers are and what they represent. Quantum numbers are mathematical equations used to describe the energy and motion of electrons in an atom. The four quantum numbers are n (principal quantum number), l (azimuthal quantum number), ml (magnetic quantum number) and ms (spin quantum number).
2. Determine the principal quantum number (n): The principal quantum number (n) is the quantum number that determines the energy level of the electron. It is always a positive integer, starting from one and increasing as the energy level increases.
3. Determine the azimuthal quantum number (l): The azimuthal quantum number (l) is the quantum number used to define the shape of the orbital in which the electron is found. It is an integer ranging from 0-n-1. It is usually designated as either s, p, d or f.
4. Determine the magnetic quantum number (ml): The magnetic quantum number (ml) is the quantum number used to define the orientation of the orbital in which the electron is found. It is an integer ranging from -l to +l.
5. Determine the spin quantum number (ms): The spin quantum number (ms) is the quantum number used to define the direction of the electron’s spin. It is either +1/2 or -1/2.
6. Calculate the total number of possible quantum numbers: Once all four of the quantum numbers have been determined, it is possible to calculate the total number of possible quantum numbers by multiplying the possible values of each quantum number together. For example, if n=3, l=2, ml=-1 to +1 and ms=+1/2 or -1/2, then the total number of possible quantum numbers is 12 (3x2x2x2).
7. Compare the results to the answer key: Finally, compare the calculated total number of possible quantum numbers to the answer key provided. If the number matches, the student has correctly identified and calculated all of the quantum numbers.
Using Visual Representations to Understand Quantum Numbers: Exploring a Quantum Numbers Worksheet
Visual representations are a powerful tool for understanding complex concepts, especially in the realm of quantum mechanics. One such representation is the quantum numbers worksheet, a graphical representation of the four quantum numbers associated with a single electron.
In the center of the quantum numbers worksheet is the atomic number, which is the number of protons in the nucleus of each atom. To the right of the atomic number is the principle quantum number, which tells us the energy level of the electron in the atom. Below the atomic number is the angular momentum quantum number, which indicates the shape of the orbital in which the electron is located. Finally, to the left of the atomic number is the magnetic quantum number, which indicates the orientation of the electron in the orbital.
Each of these quantum numbers is represented on the quantum numbers worksheet by a number from 0 to 3. As electrons move from one energy level to another, their associated quantum numbers will change. Understanding these changes can help us gain a better understanding of the behavior of electrons in an atom.
The quantum numbers worksheet also provides us with an easy way to compare the quantum numbers of different electrons. By comparing the quantum numbers of different electrons, we can gain insight into the nature of their interaction. This understanding can be used to determine the stability of different atoms and molecules and can help us understand the behavior of matter on a quantum level.
Overall, the quantum numbers worksheet is a simple but effective tool for understanding quantum numbers and the behavior of electrons in atoms. By understanding the four quantum numbers associated with a single electron, we can gain a deeper understanding of the quantum world and the behavior of matter on a quantum level.
Conclusion
The Quantum Numbers Worksheet Answers provide a good introduction to the concepts of quantum numbers and their roles in atomic structure and chemical behavior. The worksheet helps to understand the various principles associated with each of the four quantum numbers and their relationships with each other. Understanding these concepts is important for furthering our knowledge of quantum mechanics and developing an understanding of the properties of atoms.
[addtoany]