Exploring the Properties of Water: A Comprehensive Worksheet Guide
This comprehensive worksheet guide is designed to provide a thorough exploration of the properties of water. It will provide information on the physical and chemical properties of water, its structure and behavior, and the ways in which these properties affect its uses. In addition, this worksheet will explain the importance of water in the environment and in our daily lives.
Physical Properties of Water
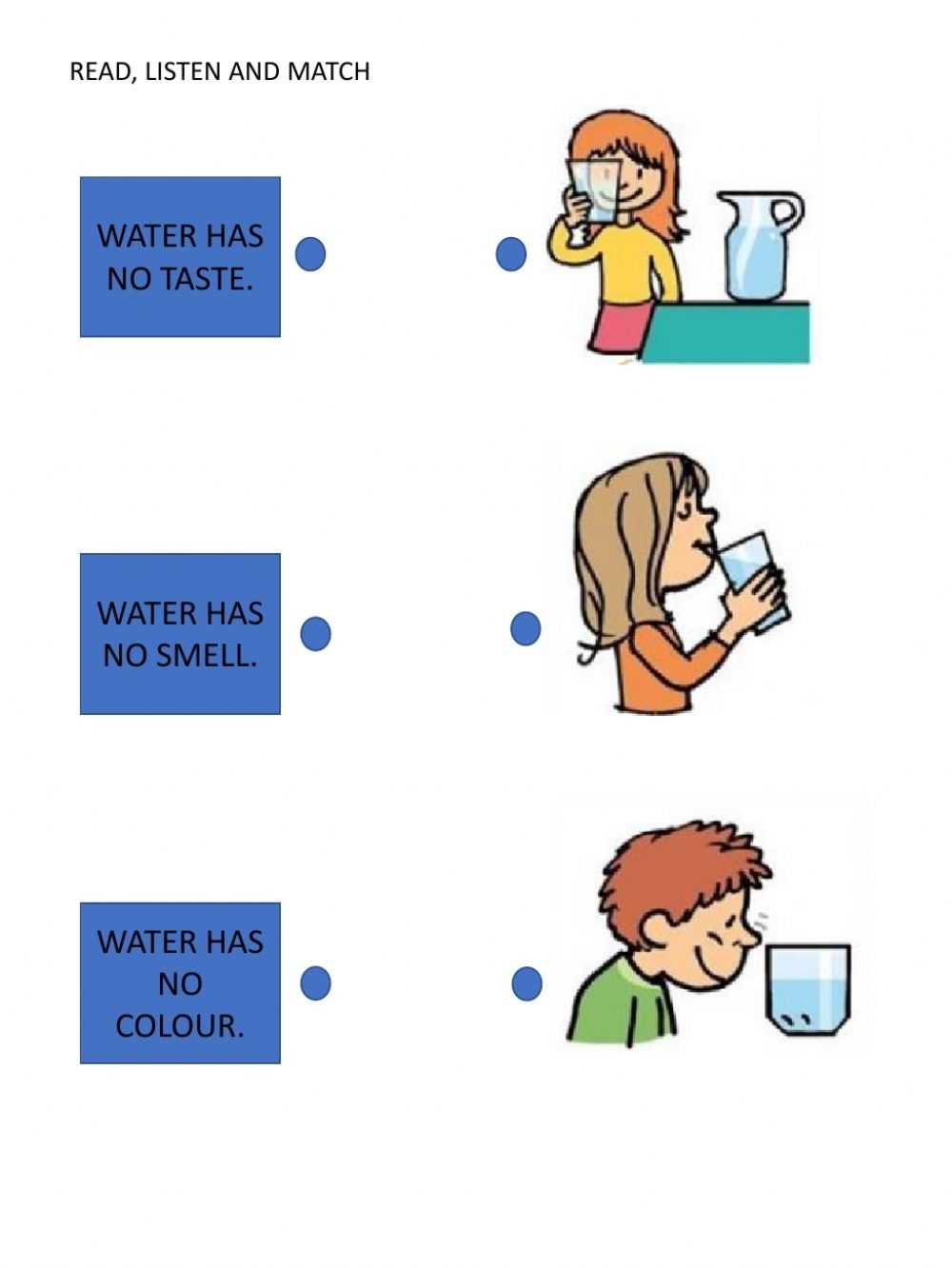
Water is a colorless, odorless liquid at room temperature. Its chemical formula is H2O, which stands for two hydrogen atoms and one oxygen atom. Water has a variety of physical properties, all of which affect its behavior in different ways.
[toc]
The boiling point of water is 100°C (212°F). The freezing point of water is 0°C (32°F). Water is most dense at 4°C (39°F). This means that water is less dense when it is a solid or a gas than when it is a liquid.
Water has a high surface tension, which is caused by the strong attraction between its molecules. This attraction causes water to form droplets on surfaces. Water is also highly cohesive, meaning that it forms a continuous film on surfaces. This property makes water an excellent solvent, allowing it to dissolve many substances.
Chemical Properties of Water
Water is a polar molecule, meaning that its positive and negative charges are not evenly distributed. This asymmetry causes water to form hydrogen bonds with other molecules, giving it a variety of chemical properties.
Water is an excellent solvent, meaning that it can dissolve many substances. This is because its polarity allows it to interact with the molecules of the substances it dissolves. Water also has a high dielectric constant, meaning that it can store and transfer electrical energy.
Water is a weak base, meaning that it can accept hydrogen ions from acids. This property allows water to buffer pH levels in the environment and in living organisms.
Structure and Behavior
Water molecules form an open lattice structure in liquid form. This structure allows water molecules to move freely, giving water its unique properties. Water molecules also form hydrogen bonds with each other, which gives water its high surface tension and cohesive properties.
Water is an unusual molecule because it is denser in its liquid form than in its solid or gaseous forms. This property causes water to expand when it freezes, which helps explain why ice floats on water.
Uses of Water
Water is essential for life on Earth. It is the basis for all biological processes and is necessary for the growth of plants and animals. Water is also used for many industrial processes, such as cooling and cleaning.
Water is also necessary for transportation and recreation. Ships and boats use water for transportation, while people use it for swimming, sailing, and other recreational activities.
Conclusion
Water is an essential component of life on Earth. Its physical and chemical properties give it a variety of uses, from sustaining life to powering industrial processes. Through this worksheet, we have explored the properties of water and the ways in which they affect its behavior.
Using the Properties of Water Worksheet to Teach Scientific Inquiry
Scientific inquiry is an important skill for students to learn, and the Properties of Water worksheet can be a valuable tool for teaching this concept. The worksheet begins with a brief introduction of the concept of scientific inquiry and the properties of water. It then moves into a series of questions on the properties of water and the scientific inquiry process.
The questions are designed to help students explore the different properties of water: boiling point, freezing point, surface tension, solubility, and viscosity. By answering these questions, students learn how to identify different properties of water, how to use the scientific method to explore these properties, and how to make predictions based on the data.
The worksheet also offers students the opportunity to practice their scientific inquiry skills. After answering the initial questions, students can then use their data to make predictions about the properties of water. This exercise encourages students to think critically and apply their knowledge to make informed decisions.
Finally, the worksheet wraps up by providing students with a summary of their findings. This allows students to review their work and reflect on their experience with the scientific inquiry process.
Overall, the Properties of Water worksheet is an effective tool for teaching scientific inquiry. By introducing students to the concept of scientific inquiry, providing them with questions to explore the properties of water, and giving them the opportunity to practice their scientific inquiry skills, this worksheet helps students develop the skills necessary for successful scientific inquiry.
Investigating the Properties of Water: A Hands-On Activity
This hands-on activity is designed to explore the properties of water. Students will be introduced to the basic physical and chemical properties of water and then use those properties to design experiments. The goal is to gain a better understanding of water and its unique properties.
The activity begins with a discussion of the different physical and chemical properties of water. Students will be asked to identify the following properties: boiling point, freezing point, viscosity, surface tension, and thermal conductivity. They will also learn about the role of water in the natural environment, including its role in the water cycle and its importance to living organisms.
Next, students will be given the opportunity to design and carry out experiments to explore the properties of water. They will be asked to use the properties they learned about in the discussion to create simple experiments or demonstrations. These experiments should focus on the physical and chemical properties of water and the effects these properties have on the environment.
The experiments should be designed so that the students can observe the effects of their actions on the water. For example, a student could design an experiment to measure the boiling point of water and observe how it changes when different temperatures are applied.
Once the experiments are complete, the students will be asked to analyze their results and discuss what they learned. This is an important part of the activity, as it encourages students to think critically and form their own conclusions about the properties of water.
By the end of the activity, students should have a better understanding of the unique properties of water and the role it plays in the environment. They should also be able to identify the physical and chemical properties of water, use them to design experiments, and analyze the results.
Analyzing the Physical and Chemical Properties of Water with a Worksheet
Water is a unique and essential molecule that is essential for life on Earth. With two hydrogen atoms covalently bonded to an oxygen atom, it is the most abundant compound on the planet. This molecule has a variety of physical and chemical properties that make it ideal for its numerous uses. To better understand this molecule, the following worksheet provides an overview of the physical and chemical properties of water.
Physical Properties
Water has a variety of physical properties that are important to its many uses. The most notable of these is its low boiling point of 212°F (100°C). This allows it to be boiled easily, making it ideal for uses such as sterilization and food preparation. Water also has a high freezing point of 32°F (0°C), making it ideal for use in refrigeration and freezing applications. Water has a relatively high boiling point compared to other substances, making it an ideal solvent for a variety of compounds.
The molecular structure of water is also quite interesting. It has a bent structure, with an oxygen atom at the center and two hydrogen atoms forming a V shape. This structure allows for strong hydrogen bonds to form between different molecules of water. This gives water an unusually high specific heat capacity, which is the amount of energy required to raise the temperature of a given mass of water by one degree Celsius. Water also has a high surface tension, allowing it to form droplets on surfaces and creating a layer of protection against dirt and bacteria.
Chemical Properties
Water is also unique in its chemical properties. It has a high degree of polarity, which means that it has a positive charge on one end and a negative charge on the other. This allows it to form strong hydrogen bonds between different molecules, giving it a high degree of cohesion. This helps it to resist evaporation and allows it to stick together as a liquid. Water is also an excellent solvent, allowing it to dissolve a variety of compounds. This property allows it to be used for a variety of applications such as cleaning and purification.
Water is also an effective buffer, meaning that it can neutralize both acids and bases. This makes it ideal for use in a variety of chemical reactions, such as the production of acids or bases. Lastly, water has a high degree of reactivity, allowing it to form a variety of compounds with other elements. This is essential for many biochemical reactions, such as the metabolism of nutrients.
In conclusion, water has a variety of physical and chemical properties that make it essential for life on Earth. Its low boiling point, high freezing point, and strong hydrogen bonds make it ideal for a variety of applications. Its polarity, solubility, buffering capacity, and reactivity also make it essential for a variety of chemical reactions. Its unique physical and chemical properties make it an invaluable resource and an essential part of life.
Conclusion
In conclusion, the Properties of Water Worksheet is a great way to learn about the various chemical, physical, and biological characteristics of water. It provides a comprehensive overview of the various properties of water, including its boiling and freezing points, surface tension, and viscosity. This worksheet can be used to help students gain a better understanding of the importance of water in the environment and how it affects the lives of all living things.
[addtoany]