How to Use Molar Mass Practice Worksheets to Master Chemistry Concepts
Molar mass practice worksheets are a great way to master the concepts of chemistry. These worksheets are designed to help students understand the relationship between elements and their molar mass. By completing a molar mass practice worksheet, students can gain a better understanding of the fundamentals of chemistry.
When using molar mass practice worksheets, it is important to understand the elements being used and their molar mass. A molar mass is the mass of one mole of a substance. The mole can be defined as the amount of material that contains 6.022 x 1023 particles. By understanding the molar mass of each element, students can better understand the relationships between elements and their mass.
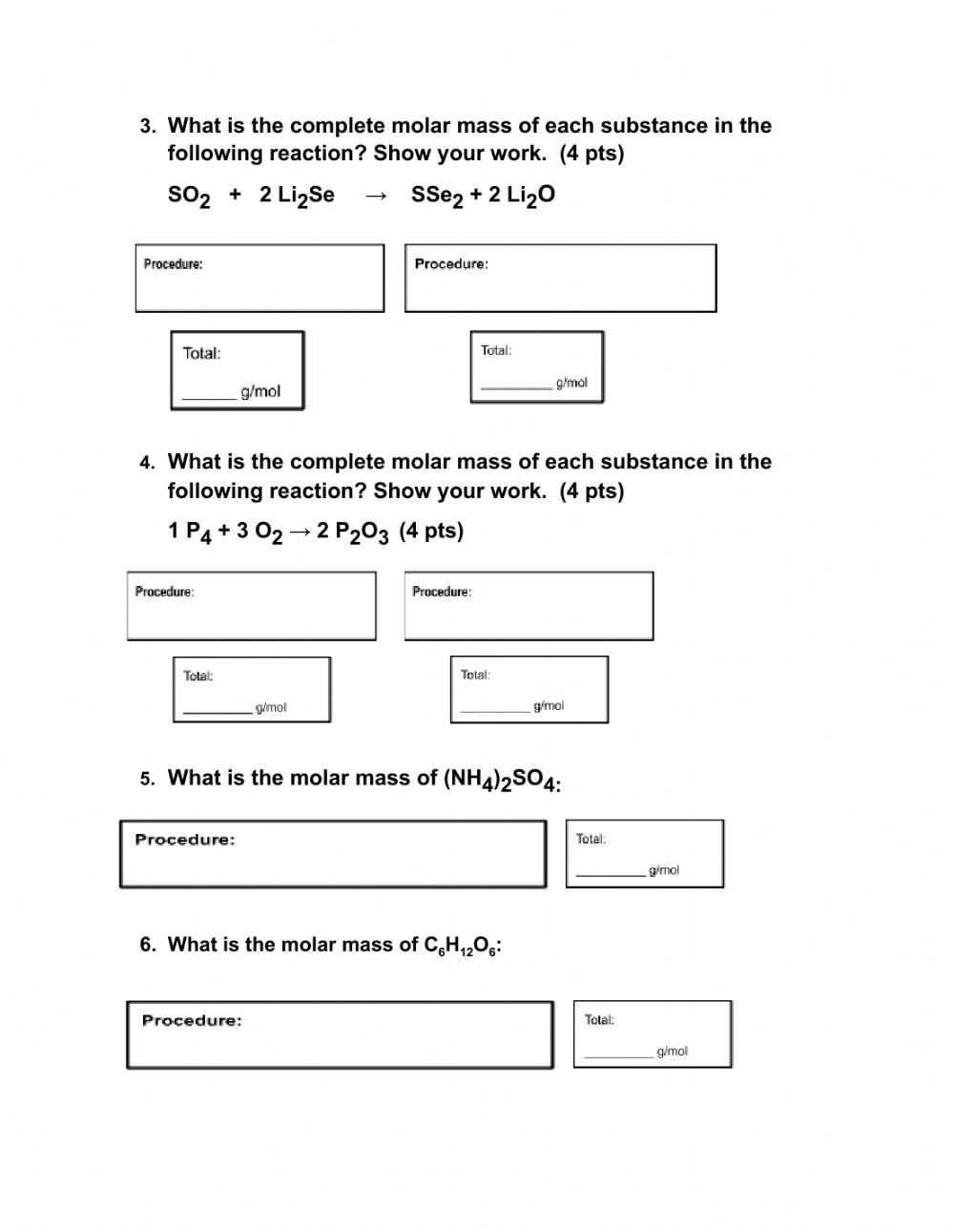
Once students are familiar with the elements and their molar mass, they can begin to complete molar mass practice worksheets. These worksheets typically provide a number of different problems and equations. These equations involve the molar mass of each element. Students must solve the equations to determine the total molar mass of the element.
[toc]
It is important to be familiar with the different types of problems on a molar mass practice worksheet. These problems involve the use of different elements and their molar mass. Students must use the molar mass to calculate the total molar mass of the elements.
Once students complete the molar mass practice worksheet, they can check their answers against the provided answers. This allows them to see if their solution was correct and if they need to try a different approach.
Molar mass practice worksheets are a great way to master the concepts of chemistry. By completing these worksheets, students can gain a better understanding of the relationships between elements and their molar mass. This knowledge can help them complete other chemistry problems with confidence.
Tips and Tricks on How to Easily Calculate Molar Masses
Calculating molar masses can be a tricky task, particularly when dealing with complex molecules. However, there are some tips and tricks to make it easier.
Firstly, it is important to understand the components of a molecule and how to determine their individual molar masses. Atomic molar masses can be gathered from the periodic table of elements, which is a useful reference tool. Once you have determined the atomic masses, you can calculate the molar mass of the molecule by adding up the individual atomic molar masses.
Additionally, a molecular formula is essential for calculating molar masses. This formula helps to provide a visual representation of the molecule, allowing you to determine the number of atoms present in the molecule and thus their individual molar masses.
It is also important to be aware of the units you are using when calculating molar masses. The commonly used unit of mass is the atomic mass unit (amu), which is equal to 1/12 of the mass of a single atom of carbon-12.
Finally, a calculator can be a great tool when calculating molar masses. Using a calculator will help you to add up the individual atomic molar masses quickly and accurately.
By following these tips and tricks, you can easily calculate molar masses and make the process much easier.
Exploring the Relationship Between Molar Mass and Chemical Reactions
The molar mass of a chemical compound is an important factor in determining how it will react with other compounds. It is the measure of the mass of one mole of a substance, and it is typically expressed in grams per mole (g/mol). The molar mass of a compound can be used to predict its reactivity with other compounds, as well as its solubility in various solvents.
In general, compounds with lower molar masses tend to have higher reactivity. This is due to the fact that molecules with lower molar masses have more energy, which makes them more likely to undergo chemical reactions. Compounds with higher molar masses, on the other hand, tend to be less reactive, as the molecules have less energy and are less likely to react.
The solubility of compounds is also affected by their molar mass. In general, compounds with higher molar masses tend to be less soluble in solvents than those with lower molar masses. This is because the molecules of higher molar mass compounds are larger and therefore less likely to dissolve in the solvent.
The molar mass of a compound can also be used to predict the rate at which a chemical reaction will occur. Compounds with higher molar masses tend to react more slowly, as the molecules have more energy and are more likely to resist the energy required for the reaction. Compounds with lower molar masses, on the other hand, tend to react more quickly due to their lower energy.
In conclusion, the molar mass of a chemical compound plays an important role in determining its reactivity, solubility, and reaction rate. By understanding the relationship between molar mass and these properties, chemists are better able to predict the behavior of compounds and design experiments accordingly.
Understanding the Different Components of a Molar Mass Practice Worksheet
The molar mass practice worksheet is an important tool for understanding the concepts of molar mass. It helps to explain the relationship between the mass of one mole of a substance and its molecular weight. The worksheet is composed of several components that must be understood in order to get the most out of it.
The first component of the worksheet is the molar mass equation. This equation helps to explain the relationship between the mass of one mole of a substance and its molecular weight. This equation is used to calculate the molar mass of a substance based on the number of atoms or molecules of that substance.
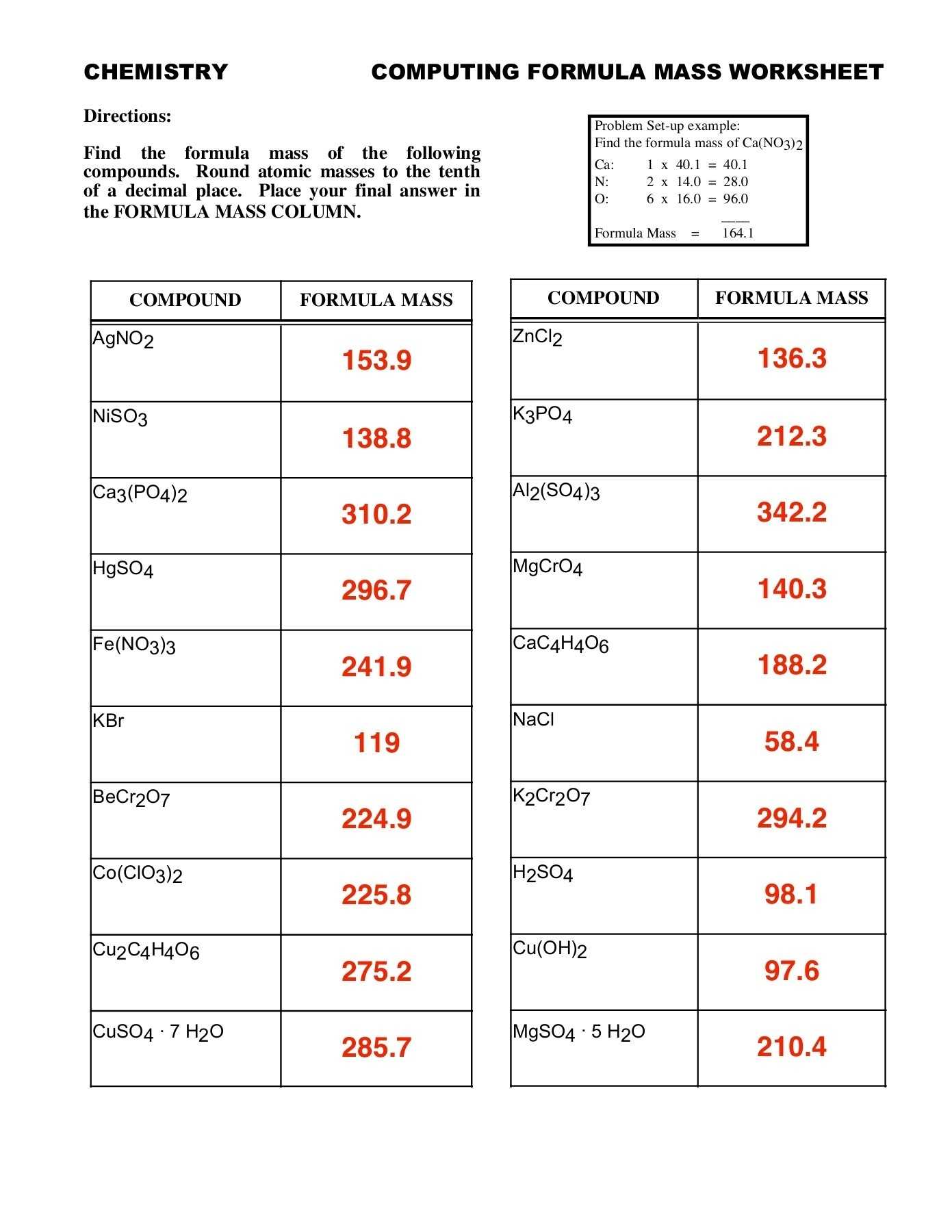
The second component of the worksheet is the molar mass table. This table helps to illustrate the relationship between the molar mass of a substance and its molecular weight. It also helps to show the differences between different substances and their respective molar masses.
The third component of the worksheet is the molecular weight calculator. This calculator helps to calculate the molecular weight of a substance based on the number of atoms or molecules of that substance. This calculator can also be used to calculate the molecular weight of a substance based on its molecular structure.
The fourth component of the worksheet is the atomic weight calculator. This calculator helps to calculate the atomic weight of a substance based on the number of atoms or molecules of that substance. This calculator can also be used to calculate the atomic weight of a substance based on its molecular structure.
The fifth component of the worksheet is the molar mass conversion chart. This chart helps to illustrate the relationship between the mass of one mole of a substance and its molecular weight. It also helps to show the differences between different substances and their respective molar masses.
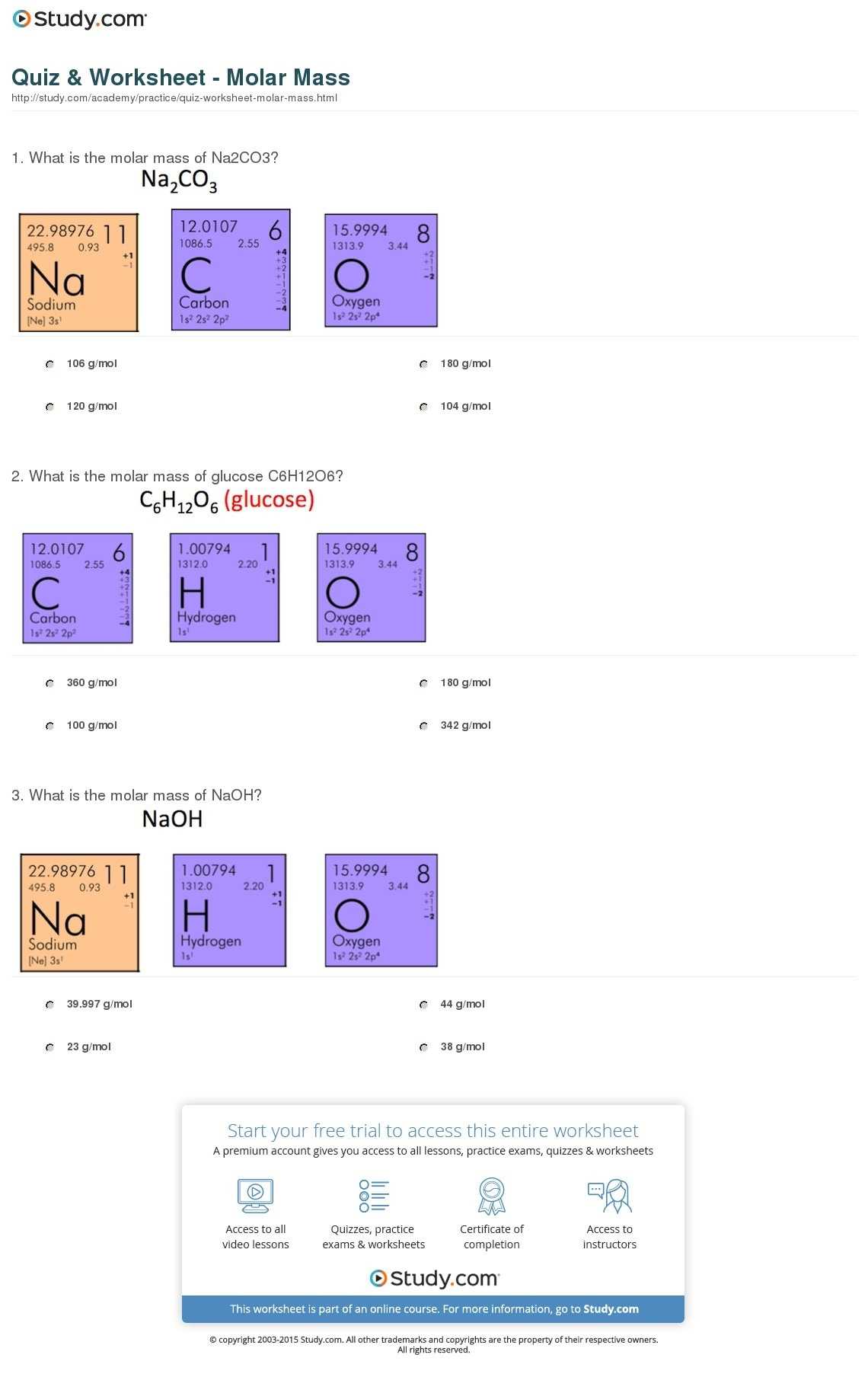
Finally, the sixth component of the worksheet is the practice problems. These problems help to reinforce the understanding of the concepts of molar mass and help to relate the concepts to real-world scenarios. By solving these problems, students can gain a better understanding of the concepts and be able to apply them in practical situations.
Conclusion
The molar mass practice worksheet was a great way to practice and review the concept of molar mass. Students were able to practice calculating the molar mass of various elements and compounds, as well as convert between mass and moles. Through completing this worksheet, students were able to gain a deeper understanding of the concept of molar mass and its applications.
[addtoany]