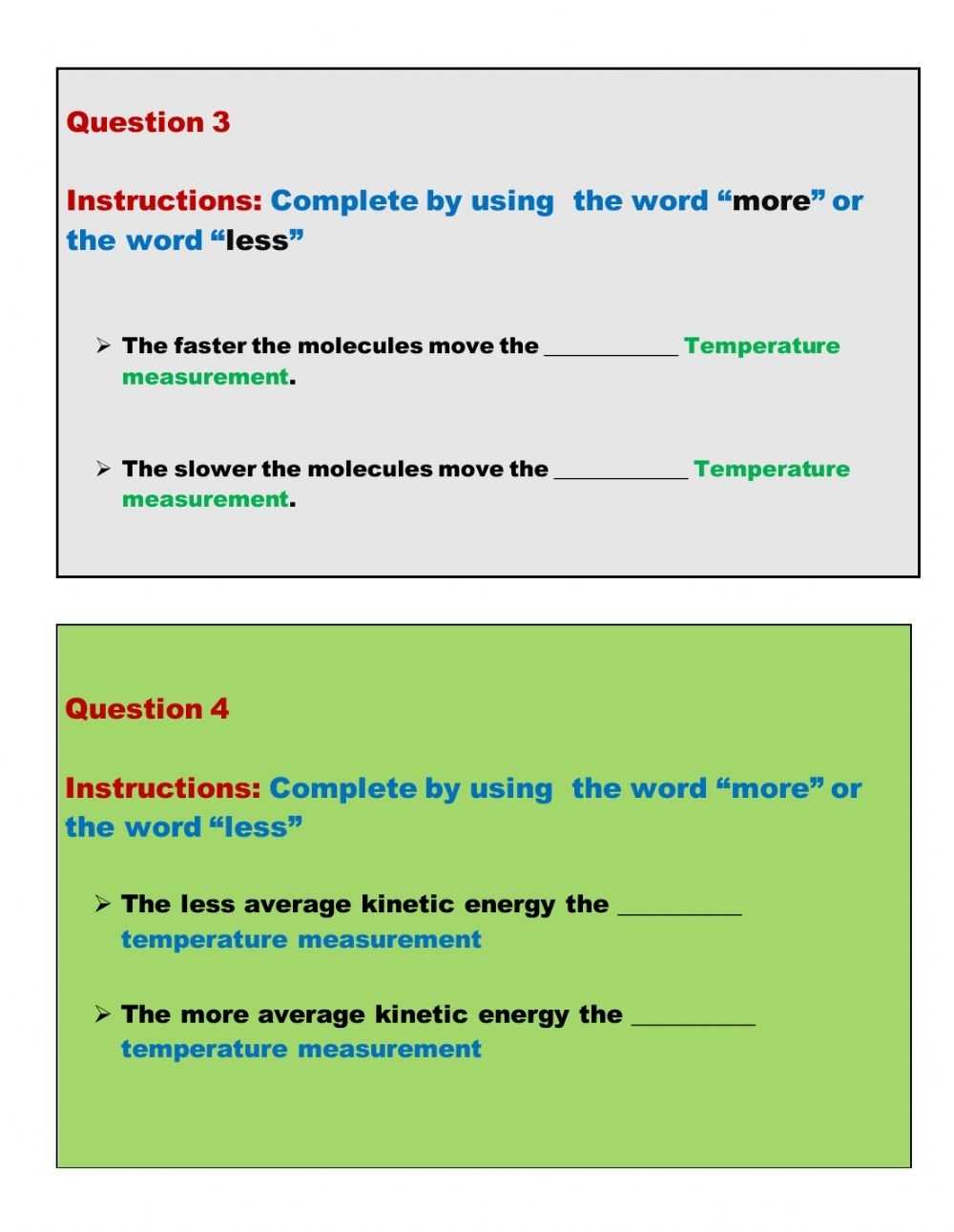
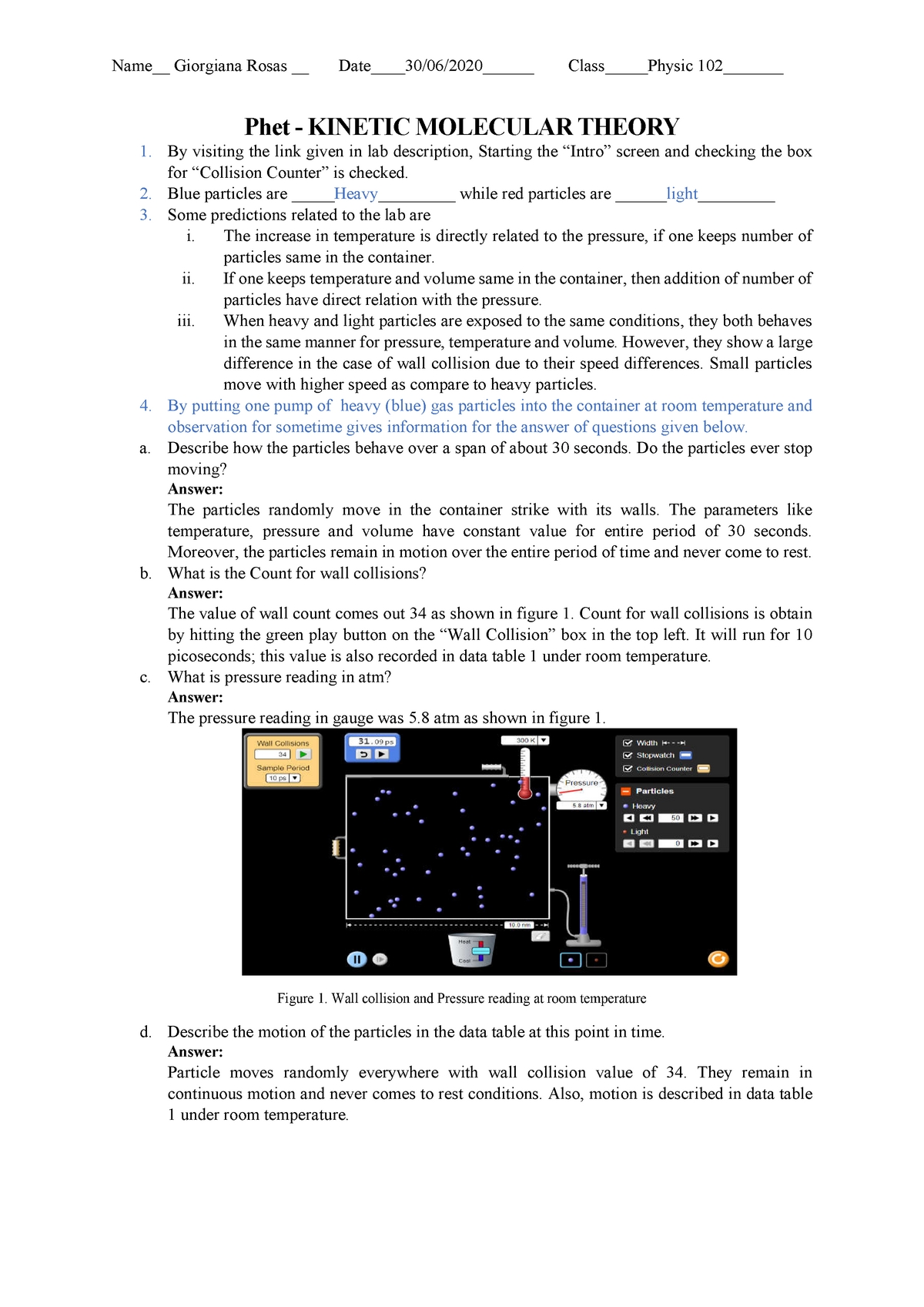
Exploring the Basics of Kinetic Molecular Theory: A Guide to Understanding the Worksheet.
Kinetic molecular theory is a fundamental concept in the field of physical chemistry. Put simply, it is an explanation of the behavior of all matter at the atomic and molecular levels. The theory helps us to understand the unique properties of different substances, including their physical states and chemical reactions.
In this guide, we will explore the basics of kinetic molecular theory and provide some tips on understanding the worksheet associated with it.
To begin, let’s define the key components of kinetic molecular theory. The first is the idea of particles in motion. This is the concept that all matter is made up of tiny particles, such as atoms and molecules, which are constantly in motion. This motion results in the various properties of a substance, such as its temperature, pressure, and viscosity.
[toc]
The second component of kinetic molecular theory is the idea of collisions. When particles collide, they interact and exchange energy. This energy exchange can result in the formation of new substances, or it can cause existing substances to break down into their components.
The third component is the idea of energy. Kinetic molecular theory states that all matter is made up of energy, and that this energy is constantly in motion. This energy is the driving force behind the properties of a substance, such as its boiling point, melting point, and other thermodynamic properties.
Now that we have a basic understanding of the components of kinetic molecular theory, let’s look at how to use it to understand the worksheet. The worksheet is designed to help students apply the principles of the theory. It will typically include questions about the properties of different substances, such as their boiling points, melting points, and other thermodynamic properties.
When attempting to answer questions on the worksheet, it is important to keep in mind the three key components of kinetic molecular theory. First, think about the particles in motion and their energy exchange. Second, consider how the energy exchange affects the properties of the substance. Finally, consider how the energy exchange between particles affects the thermodynamic properties of the substance.
By taking the time to understand the worksheet, students can gain a better understanding of how kinetic molecular theory applies to the world around them. By doing this, they can gain a better understanding of the behavior of different substances and the processes behind their properties.
Investigating the Kinetic Molecular Theory Through the Use of Worksheets.
The Kinetic Molecular Theory (KMT) is a fundamental concept of chemistry that helps scientists understand and explain the behavior of gas particles. It postulates that gas particles are in constant, random motion and are able to collide with each other and the walls of their container. The theory also states that all collisions are perfectly elastic, meaning that no kinetic energy is lost during a collision.
Worksheets can be a great tool for exploring and understanding the Kinetic Molecular Theory. By using worksheets, students can gain a better appreciation of this theory and its implications. Worksheets can be used to help students understand the concept of the KMT and the various particle behaviors associated with it, such as diffusion and effusion. Worksheets can also be used to help students visualize the motion of gas particles and their interactions with each other and their container.
Worksheets can be designed to introduce and reinforce the Kinetic Molecular Theory. For example, a worksheet can be used to introduce the concept of the KMT, explain the concept of diffusion and effusion, and provide examples of how the theory applies to everyday life. Another type of worksheet can be used to reinforce the concept of the KMT by providing students with practice questions, including questions about the behavior of gas particles and the implications of the KMT on everyday life.
Worksheets can also be used to help students explore the implications of the Kinetic Molecular Theory on other aspects of chemistry. For example, worksheets can be used to help students understand the relationship between pressure, temperature, and volume as well as the relationship between the number of particles and the pressure of a gas. Worksheets can also be used to explore the concept of the ideal gas law and its implications on chemical processes.
By using worksheets to explore and understand the Kinetic Molecular Theory, students can gain a better appreciation of this fundamental concept of chemistry. They can gain an understanding of how the KMT explains the behavior of gas particles and its implications on other aspects of chemistry, such as the ideal gas law and the relationships between pressure, temperature, and volume. Through worksheets, students can also visualize the motion of gas particles and their interactions with each other and their container.
Comparing and Contrasting the Concepts of Kinetic Molecular Theory and Gas Laws Using Worksheets.
The Kinetic Molecular Theory (KMT) and Gas Laws are two related concepts used to explain the behavior of gases. While both of these concepts are used to explain the behavior of gases, they are different in the way they are used and the types of phenomena they seek to explain.
The KMT is a microscopic theory that describes the behavior of matter at the molecular level. It is based on the idea that particles in a gas are in constant, random motion and that they interact with one another through collisions. This theory states that the pressure, temperature, and volume of a gas are related to the number of collisions the particles make with the walls of the container. It also states that the average kinetic energy of the particles is directly proportional to the temperature, and that the particles themselves do not interact with one another.
The Gas Laws, on the other hand, are macroscopic laws that are used to describe the behavior of gases on a larger scale. They are based on the idea that the pressure, temperature, and volume of a gas are related to one another. The Gas Laws can be used to predict how a gas will behave when the variables of pressure, temperature, and volume are changed. These laws are not based on any assumptions about the behavior of the particles at the molecular level, but rather on observations of the behavior of gases.
In conclusion, the Kinetic Molecular Theory and Gas Laws are two related concepts used to explain the behavior of gases. The KMT is a microscopic theory that is based on the behavior of particles at the molecular level, while the Gas Laws are macroscopic laws that are used to describe the behavior of gases on a larger scale. Both of these concepts are important in understanding the behavior of gases, and they are both useful in predicting how a gas will behave under different conditions.
Utilizing Kinetic Molecular Theory Worksheets to Explore the Properties of Gases.
Kinetic molecular theory worksheets are a great tool for exploring the properties of gases. Through the use of these worksheets, students can gain an understanding of the motion of particles and how that motion affects gas behavior.
By completing these worksheets, students will gain a better understanding of how the motion of particles in a gas affects its properties. The worksheets allow students to analyze the behavior of particles in a gas and how it can be used to explain such properties as pressure, volume, and temperature. Through this analysis, students will gain an understanding of the principles of the kinetic molecular theory and be able to apply them to the physical world.
These worksheets can also help students understand the behavior of gases in different environments. Students can explore the behavior of gases under different conditions such as pressure, temperature, and volume. By completing these worksheets, students will gain a greater understanding of how different environmental factors can affect the behavior of gases.
These worksheets can also be used to help students understand the behavior of gases in relation to other substances. Students can explore the behavior of gases when they are mixed with other substances or when they are present in a confined space. Through this exploration, students will gain an understanding of how gases interact with other substances and how this can affect their properties.
Completing kinetic molecular theory worksheets is a great way for students to gain a better understanding of the properties of gases. By exploring the behavior of particles in a gas, students will gain an understanding of how this behavior affects the properties of gases and how it can be used to explain the behavior of gases in different environments. Through this exploration, students will gain an understanding of how gases interact with other substances and how this can affect their behavior.
Examining the Relationship between Temperature and Molecular Motion Using Kinetic Molecular Theory Worksheets.
Kinetic Molecular Theory is a branch of physical chemistry that seeks to explain the behavior of matter on an atomic level. It is based on the idea that all matter is composed of small particles in constant motion, and that these particles interact with one another to produce physical changes. This theory provides a useful framework for examining the relationship between temperature and molecular motion.
The temperature of a system is directly related to the average kinetic energy of its particles. As temperature increases, the average kinetic energy of the particles increases, resulting in faster and more frequent collisions between particles. This increased motion is reflected in the characteristic properties of the material, such as its viscosity, surface tension, and vapor pressure.
This relationship between temperature and molecular motion can be explored further by completing a series of worksheets that focus on the Kinetic Molecular Theory. The worksheets can be used to identify the different components of the theory and to calculate the average kinetic energy of particles at different temperatures. They can also be used to study the behavior of gases and the effect of temperature on the rate of diffusion.
The Kinetic Molecular Theory worksheets provide an excellent opportunity to deepen students’ understanding of the relationship between temperature and molecular motion. By completing the worksheets, students will gain a greater appreciation of the molecular behavior that underlies many physical phenomena. They will also gain a better understanding of the physical properties of matter, such as its viscosity, surface tension, and vapor pressure, which are strongly influenced by temperature.
Analyzing the Effects of Pressure on Kinetic Molecular Theory Using Worksheets.
Pressure is one of the fundamental concepts of kinetic molecular theory, and its effects can be analyzed using worksheets. Pressure is the force per unit area exerted by a gas and is a measure of the number of collisions molecules have with the walls of a container. It is closely related to temperature, as the faster the molecules move, the more collisions they have with the walls and thus, the greater the pressure.
When analyzing the effects of pressure on kinetic molecular theory with worksheets, the student should first identify the variables that affect the pressure of a gas. These include the number of molecules, the volume of the container, the temperature of the gas, and the mass of the molecules. The student should then identify the relationships between these variables and pressure. For instance, the pressure of a gas is inversely proportional to its volume, meaning that the pressure increases as the volume decreases. The student should also be aware that the pressure of a gas is directly proportional to its temperature, meaning that an increase in temperature will result in a corresponding increase in pressure.
Once the student has identified the variables that affect pressure and the relationships between them, the next step is to use worksheets to calculate the values of pressure for a given set of variables. To do this, the student should enter the values of the variables into the worksheet and then use the equations provided to calculate the pressure. The student should also pay attention to the units used in the equations to ensure accuracy.
Finally, the student should use their calculations to draw conclusions about the effects of pressure on kinetic molecular theory. For instance, they can determine whether pressure increases or decreases with the volume of a gas and with temperature. They can also explore the relationships between different variables to determine how they influence the pressure of a gas. By doing this, the student can gain a better understanding of pressure and its effects on kinetic molecular theory.
Exploring the Role of Intermolecular Forces in Kinetic Molecular Theory Using Worksheets.
Intermolecular forces play a key role in the Kinetic Molecular Theory, which is used to explain the behavior of gases and other particulate systems. These forces, which include van der Waals, hydrogen bonding, and dipole-dipole interactions, are responsible for the attraction and repulsion of molecules in a given system. By understanding the role of these forces, one can better comprehend the behavior of gases and other particulate systems.
In order to help students learn about the role of intermolecular forces in kinetic molecular theory, worksheets can be used to help students explore the concept. These worksheets can be used to help students understand what intermolecular forces are and how they can affect the behavior of molecules in a system. Through the use of these worksheets, students can gain an understanding of the different types of intermolecular forces, their strengths, and how they can affect the behavior of molecules in a system.
The worksheets can also be used to explore the relationship between intermolecular forces and other concepts, such as temperature and pressure. Students can use the worksheets to explore how different forces can affect the behavior of molecules in a system. Additionally, students can explore how different intermolecular forces can affect the properties of a system, such as its rate of diffusion and its ability to hold onto its energy.
By using worksheets, students can gain a better understanding of the role of intermolecular forces in kinetic molecular theory. Through their exploration, students can learn how intermolecular forces affect the behavior of molecules in a system and how different forces can affect the properties of a system. By using worksheets, students can gain a better understanding of the concept and can be better prepared to apply it to their studies.
Applying Kinetic Molecular Theory to the Study of Chemical Reactions with Worksheets.
The Kinetic Molecular Theory (KMT) is a tool used to help explain the behavior of particles in a chemical reaction. It is based on the idea that all matter is composed of tiny particles that are in constant motion. These particles are affected by collisions, temperature, and pressure. By using this theory, scientists can gain insight into the behavior of particles during a chemical reaction.
The KMT can be applied to the study of chemical reactions in a variety of ways. For example, it can be used to explain the rate at which particles react with one another. It can also be used to explain the distribution of energy among particles in a reaction. Furthermore, the KMT can be used to understand how different conditions, such as temperature and pressure, affect a reaction.
In order to take full advantage of the KMT’s potential, it is important to understand the different worksheets that are used. These worksheets are designed to help students visualize the behavior of particles in a reaction. They usually involve diagrams or equations that represent the various elements of a reaction. By studying these worksheets, students can gain a better understanding of how the KMT applies to a reaction and how it can be used to explain the behavior of particles.
The Kinetic Molecular Theory can be a powerful tool for the study of chemical reactions. By using worksheets, students can gain an enhanced understanding of how the KMT works and how it can be used to explain the behavior of particles during a reaction. With this knowledge, students can gain a better appreciation for the complexities of chemical reactions and how they can be explained and manipulated.
Conclusion
The Kinetic Molecular Theory Worksheet provides an excellent introduction to the basic concepts of the Kinetic Molecular Theory. It provides an opportunity to gain a better understanding of the behavior of gases, the relationship between temperature and pressure, and the effects of collisions on molecules. It also provides a foundation for further study of physical chemistry and thermodynamics. By understanding the basic principles of the Kinetic Molecular Theory, students will be able to better understand the behavior of gases and the properties of matter.
[addtoany]