Explaining the Ideal Gas Laws: A Step-by-Step Guide to Working with an Ideal Gas Laws Worksheet
The Ideal Gas Laws provide a convenient way for scientists to accurately predict the behavior of gases. To accurately use these laws, it is important to have a thorough understanding of the fundamentals and to be able to accurately complete an Ideal Gas Laws Worksheet. This guide will provide step-by-step instructions for working through an Ideal Gas Laws Worksheet.
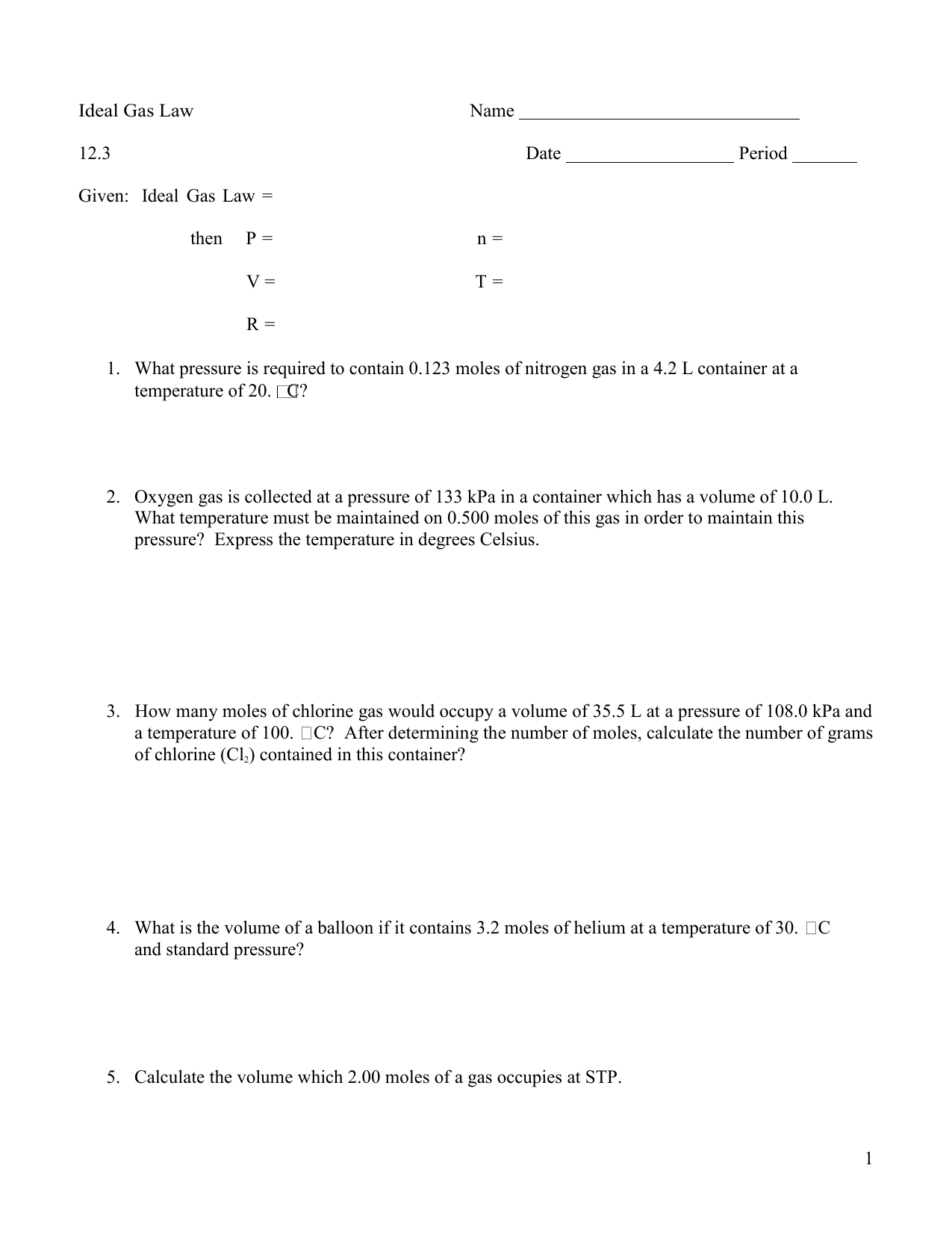
The first step is to become familiar with the three Ideal Gas Laws. These laws state that a gas’s pressure, volume, and temperature are all related. The Ideal Gas Law can be written in the form PV = nRT, where P is the pressure, V is the volume, n is the number of moles of gas, R is the gas constant, and T is the temperature.
The next step is to fill out the Ideal Gas Laws Worksheet. This worksheet will ask for values of pressure, volume, temperature, and the number of moles of gas. It is important to make sure that the units for each are consistent. For instance, if pressure is given in atmospheres, then temperature should also be given in units of Kelvin.
[toc]
Once the data for the Ideal Gas Laws Worksheet has been obtained, the next step is to solve for the unknowns. This is done by rearranging the Ideal Gas Law equation to solve for the desired quantity. For instance, if the pressure and temperature are known, then the equation can be rearranged to solve for volume. If the volume, temperature, and number of moles of gas are known, then the equation can be rearranged to solve for pressure.
Finally, the Ideal Gas Laws Worksheet can be used to determine the behavior of a gas given certain conditions. Knowing how pressure, volume, temperature, and the number of moles of gas are related can help scientists predict how a gas will behave in a given situation.
Using the Ideal Gas Laws Worksheet can be a powerful tool for scientists to accurately predict the behavior of gases. By following the steps outlined in this guide, scientists can be confident that they are accurately working with an Ideal Gas Laws Worksheet.
Analyzing Real-World Examples with an Ideal Gas Laws Worksheet
The Ideal Gas Laws Worksheet is a valuable tool for analyzing real-world examples of gas behavior. This worksheet allows for the evaluation of different scenarios, enabling a comprehensive understanding of the behavior of an ideal gas.
The Ideal Gas Laws Worksheet is a systematic approach to problem solving, providing students with the ability to apply their knowledge of the ideal gas laws to real-world scenarios. The worksheet is divided into three columns; the first column is used to enter the given values, the second column is used to calculate the unknowns, and the third column is used to show the results.
The first step in using the Ideal Gas Laws Worksheet is to enter the given values into the first column. Depending on the scenario, this could include the temperature, pressure, volume, or mass of the gas. Once the given values have been entered, the next step is to calculate the unknowns. This calculation is done by using the ideal gas laws, which state that when pressure, temperature, and volume are held constant, the mass of a gas is proportional to its moles.
Once the unknowns have been calculated, the results can be entered into the third column. This column is used to show the results of the calculations. The results can be compared to the original given values to determine if the ideal gas laws have been correctly applied.
The Ideal Gas Laws Worksheet is a great tool for gaining a better understanding of the behavior of an ideal gas in various real-world scenarios. With this worksheet, students are able to apply their knowledge of the ideal gas laws and analyze real-world examples. The worksheet is a valuable tool for understanding the behavior of an ideal gas, and it provides students with the ability to apply their knowledge and understand real-world scenarios.
How to Use the Ideal Gas Laws to Estimate the Volume of a Gas
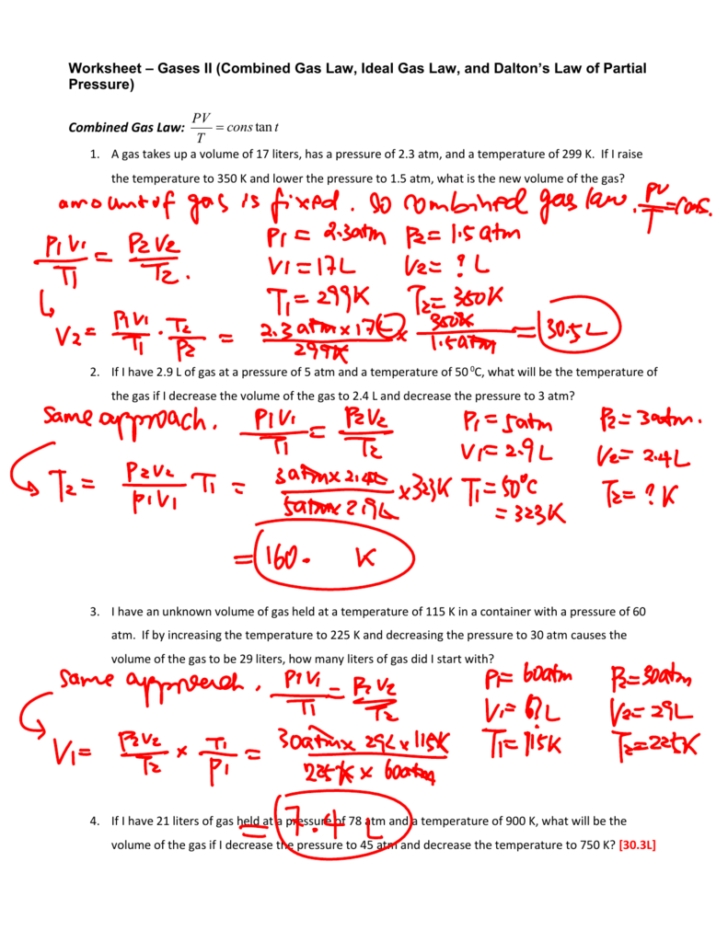
The Ideal Gas Laws are a set of equations used to calculate the properties of an ideal gas. They can be used to estimate the volume of a gas by combining the ideal gas equation with other equations from this set.
The first step is to calculate the number of moles of gas present. This can be done by dividing the pressure of the gas (in atmospheres) by the product of its temperature (in Kelvin) and the gas constant (which is equal to 0.0821 L atm/mol K). This gives the number of moles of gas present.
The next step is to use the ideal gas equation to calculate the volume of the gas. This equation states that the product of pressure, volume and temperature (in Kelvin) is equal to the number of moles of gas present multiplied by the gas constant. Therefore, by rearranging the equation, the volume of the gas can be calculated by dividing the product of pressure and temperature by the number of moles of gas present and the gas constant.
Finally, the volume of the gas can be converted to the desired units if necessary. This is done by multiplying the volume in litres by the conversion factor for the desired unit. For example, to convert the volume to cubic metres, the volume in litres should be multiplied by 0.001.
By following these steps, the volume of a gas can be estimated using the Ideal Gas Laws.
Introducing the Ideal Gas Laws: A Comprehensive Overview of the Key Concepts Covered in an Ideal Gas Laws Worksheet
The Ideal Gas Laws are a set of fundamental relationships that help to explain the behavior of gases. They were first proposed by a famous Scottish physicist and chemist, James Clerk Maxwell, in 1873. Maxwell’s work was based on the work of other scientists, including John Dalton and his law of partial pressures. The Ideal Gas Laws are useful for predicting a wide range of properties of gases, such as temperature, pressure, and volume.
The Ideal Gas Laws are composed of three main equations. The first, known as the ideal gas equation, states that the pressure and temperature of a gas are inversely proportional to its volume. This means that as pressure increases, the volume decreases and vice versa. The second equation, known as the ideal gas law constant, states that for a given amount of gas, the pressure, temperature and volume are all proportional. The final equation, known as the ideal gas law, states that the product of the pressure and volume of a gas is equal to the product of the temperature and the ideal gas law constant.
A worksheet on the Ideal Gas Laws can help to reinforce these concepts. The worksheet should provide an overview of the basic equations and definitions related to the Ideal Gas Laws, such as the ideal gas equation, the ideal gas law constant, and the ideal gas law. It should also include examples and practice problems that demonstrate how to apply the equations in real-world scenarios.
The Ideal Gas Laws are important for understanding the behavior of gases, and a worksheet can help to provide a comprehensive overview of the key concepts. By exploring the equations, definitions, and examples, students can gain a better understanding of the laws and how to apply them in problem-solving scenarios.
Conclusion
In conclusion, the Ideal Gas Laws Worksheet is a great tool to help students learn and understand the principles of ideal gas law and its various equations. It provides a comprehensive overview of the various equations, such as the ideal gas law and the ideal gas equation, and helps students understand how they are related to one another and how to use them to solve problems. The worksheet also provides practice problems that allow students to apply their knowledge of the ideal gas laws to real-world scenarios. Overall, the Ideal Gas Laws Worksheet is a valuable resource for students looking to learn more about the ideal gas laws.
[addtoany]