Exploring the Different Types of Solutions in Elements Compounds Mixtures Worksheet Answers
Elements, compounds, and mixtures are the three main types of solutions that are often studied in chemistry. Each type of solution has its own distinct characteristics that are important to understand in order to understand the different types of solutions.
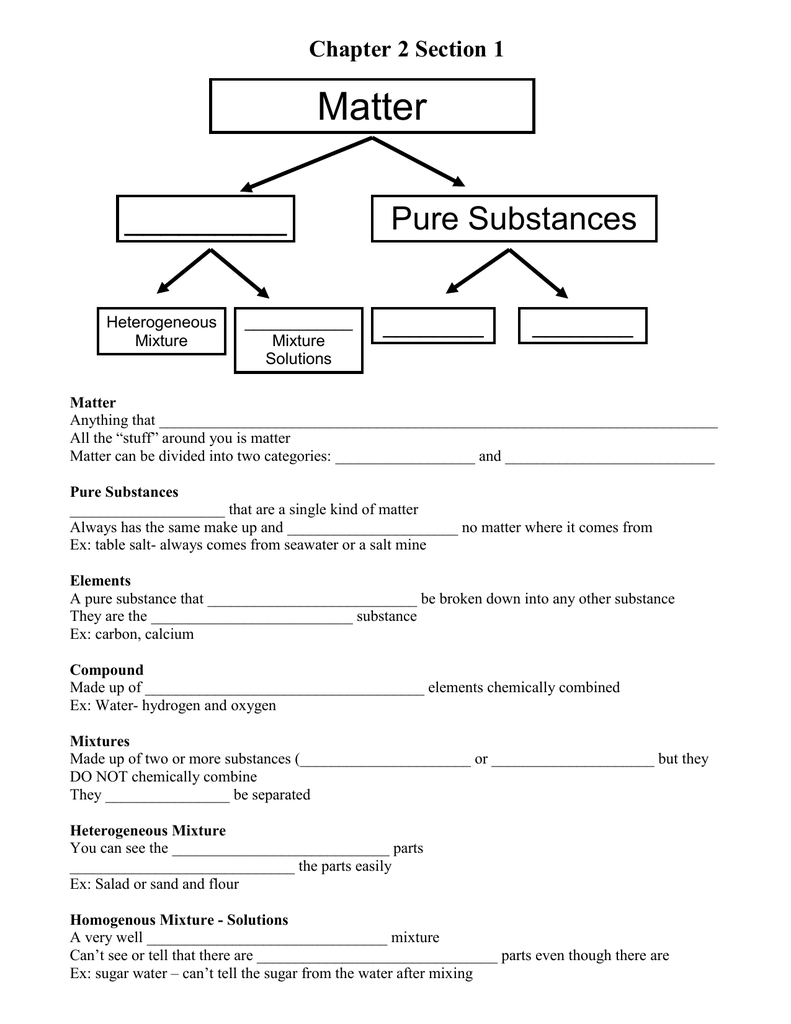
Elements are the simplest type of solution and can be found in their pure form. They are composed of just one type of atom and cannot be broken down further. Examples of elements include oxygen, hydrogen, carbon, and nitrogen.
Compounds are composed of two or more different types of atoms. The atoms are bonded together in a specific ratio and are not able to be separated easily. Examples of compounds include water, salt, glucose, and ethanol.
[toc]
Mixtures are solutions that contain two or more different types of substances, where each substance retains its individual properties. Mixtures can be divided into two categories: homogeneous and heterogeneous. Homogeneous mixtures are those in which the components are uniformly distributed. Examples of homogeneous mixtures are air, seawater, and sugar in water. Heterogeneous mixtures, on the other hand, have components that are visibly distinct. Examples of heterogeneous mixtures include sand, carbon dioxide, and iron filings in water.
In conclusion, elements, compounds, and mixtures are the three main types of solutions that are studied in chemistry. Understanding the characteristics of each type of solution can help in understanding the different types of solutions and their applications.
An Overview of the Scientific Method Used in Elements Compounds Mixtures Worksheet Answers
The scientific method is an organised approach to answering a question or solving a problem. It is the most widely used approach in scientific research, and is the cornerstone of the scientific process. The scientific method can be used to answer questions or solve problems related to elements, compounds, and mixtures. This worksheet provides a detailed overview of the scientific method and how it can be used in relation to elements, compounds, and mixtures.
The scientific method consists of five steps: observation, hypothesis, experimentation, analysis, and conclusion. In the observation step, the researcher observes the phenomenon that they are studying, and collects data. In the hypothesis step, the researcher formulates a hypothesis that explains the phenomenon. In the experimentation step, the researcher tests the hypothesis by conducting experiments and collecting data. In the analysis step, the researcher analyses the data collected from the experiments and draws conclusions based on the results. Finally, in the conclusion step, the researcher summarizes the results and draws a conclusion about the hypothesis.
To use the scientific method in relation to elements, compounds, and mixtures, the researcher first needs to make an observation about the phenomenon that they are studying. For example, if the researcher is studying the properties of a particular compound, they will observe its chemical and physical properties. From the observation, the researcher will then form a hypothesis about the properties of the compound. This hypothesis can then be tested by conducting appropriate experiments to see if it is correct. The results of these experiments can then be analysed to draw conclusions about the properties of the compound.
The scientific method can also be used to answer questions or solve problems related to elements, compounds, and mixtures. For example, if the researcher is trying to identify the components of a particular mixture, they will first observe the mixture and collect data. They will then form a hypothesis about the components of the mixture and conduct experiments to test the hypothesis. The results of the experiments can then be analysed to draw conclusions about the components of the mixture.
The scientific method is an invaluable tool for scientists and can be used to answer questions or solve problems related to elements, compounds, and mixtures. By following the five steps of the scientific method, researchers can accurately and reliably answer questions or solve problems related to elements, compounds, and mixtures.
Investigating the Different Types of Chemical Reactions in Elements Compounds Mixtures Worksheet Answers
Chemical reactions are a fundamental part of chemistry, and they are responsible for transforming the chemical composition of matter. In this worksheet, we will be exploring the different types of chemical reactions that occur in elements, compounds and mixtures.
1. What is a chemical reaction?
A chemical reaction is a process whereby one or more substances are changed into one or more other substances. Chemical reactions involve the rearrangement of atoms and molecules, resulting in the formation of new substances. Chemical reactions are driven by energy changes, either releasing or absorbing energy.
2. What is the difference between elements, compounds and mixtures?
An element is a pure substance that cannot be broken down into simpler substances by chemical or physical means. A compound is a combination of two or more elements in a fixed ratio. A mixture is a combination of two or more substances which are not chemically combined and can be easily separated.
3. What types of chemical reactions occur in elements?
Chemical reactions in elements involve the formation or breaking of chemical bonds. For example, when two elements combine, the atoms of each element join together to form a new compound. This is known as a combination reaction. Elements can also undergo decomposition reactions, in which a compound is broken down into its constituent elements.
4. What types of chemical reactions occur in compounds?
Chemical reactions in compounds involve the formation or breaking of chemical bonds. Compounds can undergo combination reactions, in which two or more molecules combine to form a new compound. They can also undergo decomposition reactions, in which a compound is broken down into its constituent elements. Additionally, compounds can undergo substitution reactions, in which one atom or group of atoms is replaced by another atom or group of atoms.
5. What types of chemical reactions occur in mixtures?
Chemical reactions in mixtures involve the formation or breaking of chemical bonds. Mixtures can undergo combination reactions, in which two or more molecules combine to form a new compound. They can also undergo decomposition reactions, in which a compound is broken down into its constituent elements. Additionally, mixtures can undergo single-replacement reactions, in which an element in a compound is replaced by another element.
Understanding the Role of Molecular Substructure in Elements Compounds Mixtures Worksheet Answers
The role of molecular substructure in elements, compounds, and mixtures is an important concept to understand in order to properly identify different types of substances. Molecular substructure refers to the arrangement of atoms within a molecule, which is the smallest unit of a substance that still retains its chemical properties. In elements, the molecules are composed of only one type of atom, whereas in compounds, the molecules are made up of two or more types of atoms. Mixtures, on the other hand, can consist of elements, compounds, or both.
The molecular substructure of an element or compound determines its physical and chemical properties. For example, the boiling point of a substance will depend on the type of bonds formed between the atoms in its molecular substructure, such as single, double, or triple bonds. Additionally, the type of bonds formed between the atoms will determine the type of reaction a substance can undergo. For example, elements with single bonds tend to react with other elements more easily than those with double or triple bonds.
The molecular substructure of a mixture, however, is not as defined as that of an element or compound. Mixtures are made up of two or more substances that are physically combined, but not chemically bonded. This means that the properties of the mixture are determined by the properties of the individual substances that make up the mixture. For example, the boiling point of a mixture may be lower than that of one of the substances in the mixture due to the presence of other substances with lower boiling points.
In conclusion, the role of molecular substructure in elements, compounds, and mixtures is essential in understanding the physical and chemical properties of substances. Elements are composed of only one type of atom, whereas compounds are made up of two or more types of atoms and mixtures are physically combined but not chemically bonded. The type and arrangement of atoms within the molecules of elements or compounds will determine the physical and chemical properties of the substance, whereas the properties of a mixture will be determined by the properties of the individual substances that make up the mixture.
Conclusion
The Elements, Compounds, and Mixtures Worksheet Answers provides a great opportunity for students to develop their understanding of the differences between elements, compounds, and mixtures. By completing the worksheet, students can gain a better understanding of the various components that make up the various substances and how they can interact with one another. As such, the Elements, Compounds, and Mixtures Worksheet Answers can help students develop a better understanding of the material they are studying and become more confident in their understanding of chemistry.
[addtoany]