Exploring the Types of Covalent Bonding: A Guide to Understanding the Covalent Bonding Worksheet Answer Key
Covalent bonding is a type of chemical bonding that occurs between two atoms when they share electrons. This bond is formed when the electrons of the two atoms become attracted to the nuclei of both atoms. The strong attractive force between the two nuclei holds the atoms together and forms a covalent bond.
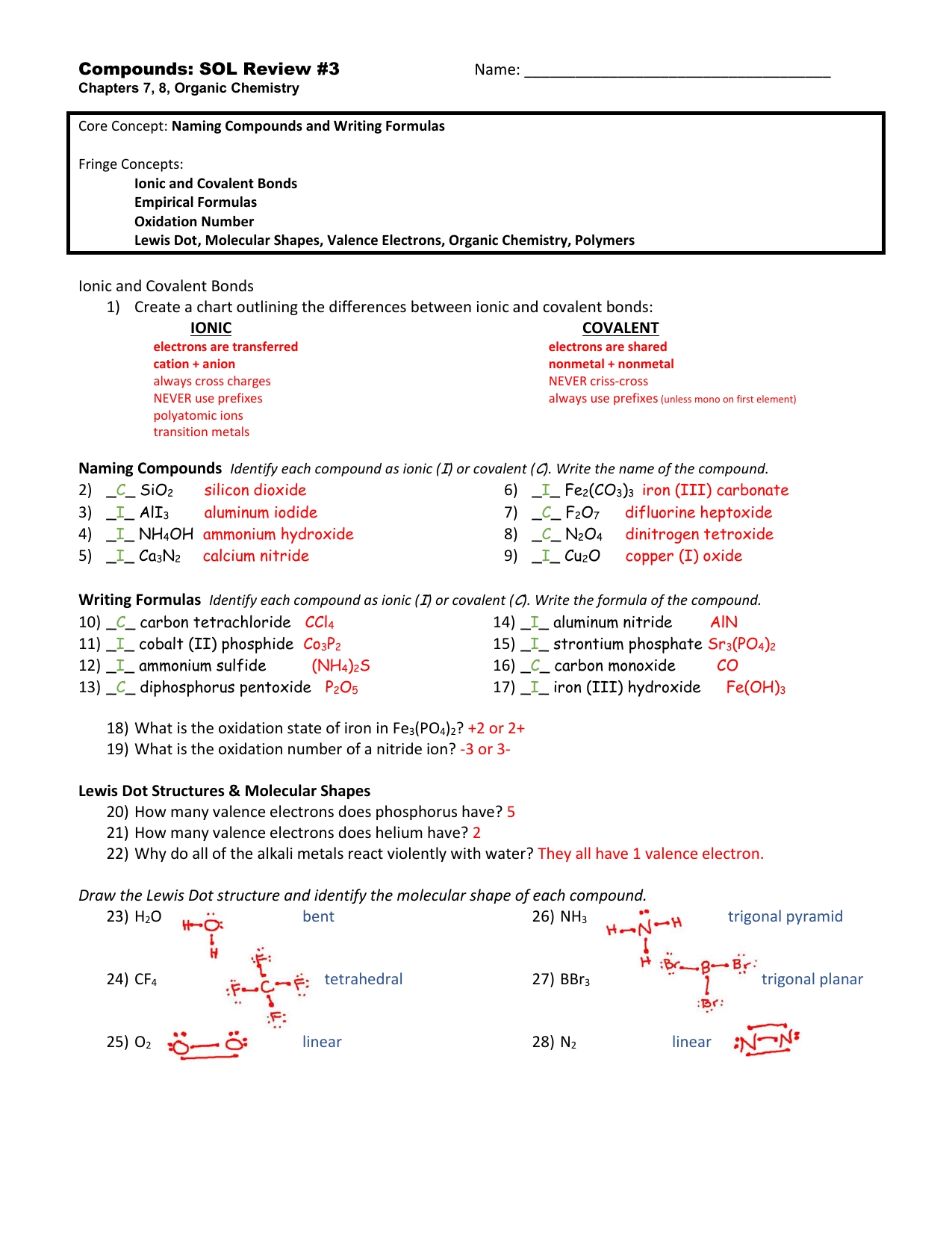
There are two types of covalent bonding: polar covalent bonds and nonpolar covalent bonds. Polar covalent bonds occur when the two atoms that are sharing electrons have different electronegativity values. Electronegativity is a measure of how strongly an atom attracts electrons. In a polar covalent bond, one atom will have a higher electronegativity than the other and will attract more electrons from the other atom. The result is a polar molecule with a partial negative charge on the more electronegative atom and a partial positive charge on the less electronegative atom.
Nonpolar covalent bonds occur when the two atoms that are sharing electrons have the same electronegativity values. In this case, both atoms will attract the same amount of electrons from each other and will form a molecule with no partial positive or negative charges.
[toc]
In addition, covalent bonds can be further classified into single, double, or triple bonds. A single bond occurs when two atoms share one pair of electrons, a double bond occurs when two atoms share two pairs of electrons, and a triple bond occurs when two atoms share three pairs of electrons. It is important to note that the more electrons that are shared between two atoms, the stronger the bond is.
In summary, covalent bonding is a type of chemical bonding that occurs between two atoms when they share electrons. There are two types of covalent bonds: polar and nonpolar. Polar covalent bonds occur when the two atoms have different electronegativity values while nonpolar covalent bonds occur when the two atoms have the same electronegativity values. In addition, covalent bonds can also be further classified into single, double, or triple bonds depending on how many pairs of electrons are shared between the two atoms.
How to Interpret the Covalent Bonding Worksheet Answer Key for Maximum Understanding
Interpreting the Covalent Bonding Worksheet Answer Key is an important step in understanding the fundamentals of covalent bonding. To properly interpret the answer key, it is necessary to be familiar with the concepts and terms associated with this type of bonding.
The answer key contains a list of questions and answers related to covalent bonding. It is important to read through the questions carefully to ensure that the answers make sense in the context of the question. Each answer should be read thoroughly to ensure that all of the relevant information has been considered. Additionally, the answer key may contain diagrams and visual aids. These diagrams should be studied carefully to ensure that the correct information is being conveyed.
After reading through the answer key, it is important to review the answers to ensure that they are accurate and consistent with the principles of covalent bonding. Additionally, it is beneficial to review the definitions of key terms as outlined in the answer key to ensure that they are being correctly applied to the questions.
Finally, it is important to take the time to consider the implications of the answer key and how it can be applied to real-world scenarios. By doing this, it is possible to gain a deeper understanding of covalent bonding and how it works.
A Step-by-Step Guide to Solving Covalent Bonding Problems Using the Covalent Bonding Worksheet Answer Key
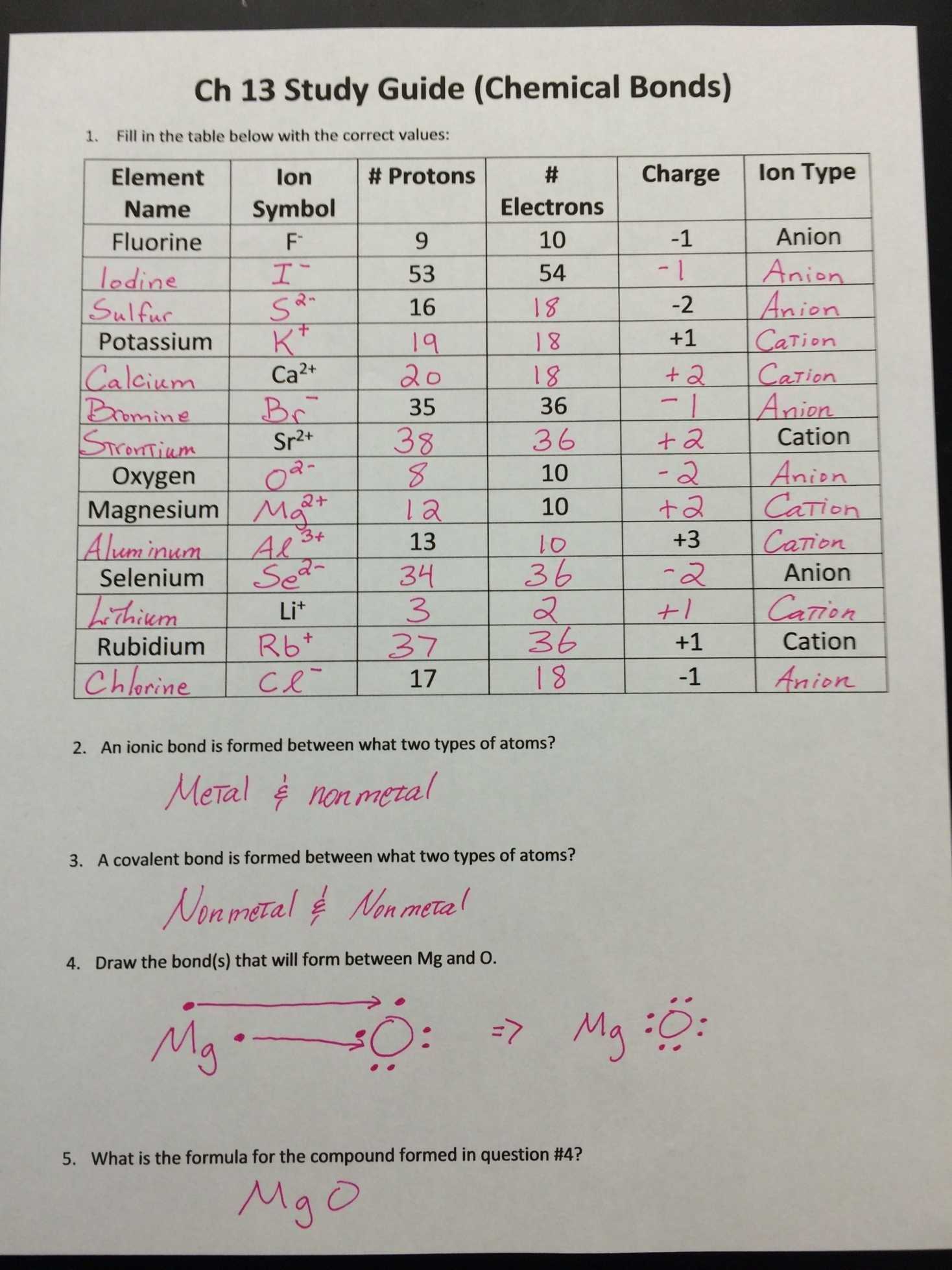
Step 1: Read and understand the problem. Carefully read the problem to determine its purpose and the concepts that need to be applied. Take note of any information that is provided, such as the elements and their respective symbols.
Step 2: Gather the necessary information. Refer to a periodic table to acquire the atomic number and electron configurations for the elements involved in the problem.
Step 3: Calculate the valence electrons for each element. Calculate the number of valence electrons for each element by subtracting the atomic number from the total number of electrons in the outermost shell.
Step 4: Use the Covalent Bonding Worksheet Answer Key to determine the number of covalent bonds formed. The answer key is a chart that shows the number of covalent bonds that can be formed between two elements based on their respective valence electrons.
Step 5: Draw the Lewis dot structures. Using the answer key, draw the Lewis dot structures for each element and the bonds that are formed.
Step 6: Calculate the bond order. Calculate the bond order by counting the number of bonding electrons and the number of non-bonding electrons.
Step 7: Determine the type of bond formed. Use the bond order to determine the type of bond formed, such as single, double, or triple covalent bond.
Step 8: Calculate the bond length. Calculate the bond length using the bond order and the bond length equation.
Step 9: Analyze the results. Compare the calculated results to the expected results to determine whether the problem has been solved correctly.
Step 10: Repeat the process. If necessary, repeat the steps above for any other elements involved in the problem.
Unraveling the Mysteries of Covalent Bonding: A Look at the Covalent Bonding Worksheet Answer Key
Covalent bonding is a fundamental process that occurs in every molecule. It is responsible for the stability of molecules and their unique properties. Despite its importance, many of the specifics of covalent bonding remain a mystery. This covalent bonding worksheet answer key is designed to help unravel some of these mysteries by providing detailed explanations of how covalent bonding works.
The answer key begins by providing a definition of covalent bonding and its importance. It then describes the four different types of covalent bonds, including single, double, triple, and quadruple bonds. It explains the structure and properties of each of these bonds and provides examples of how they are formed.
The answer key then outlines the two types of covalent compounds, ionic compounds and non-ionic compounds, and explains the differences between them. It then discusses the differences between organic and inorganic compounds, and how they interact with one another.
The answer key then explains the concept of resonance and its importance in covalent bonding. It then outlines the different types of molecular orbitals, including sigma and pi orbitals, and how they interact with one another. Finally, it discusses the types of molecular shapes, including tetrahedral, trigonal planar, and linear, and how they affect covalent bonding.
By providing a detailed explanation of the different aspects of covalent bonding, this answer key helps to unravel the mysteries of covalent bonding and provides students with the knowledge needed to understand this important process.
Conclusion
The Covalent Bonding Worksheet Answer Key provides students with a clear understanding of the concept of covalent bonding and how it relates to the structure of molecules. Through the use of the worksheet, students can gain a better understanding of the principles of covalent bonding and how they impact the properties of molecules. It is a valuable tool to help students gain a better understanding of the subject matter.
[addtoany]