Exploring the Nature of Ionic Bonds: What You Need to Know
Ionic bonds form when atoms of different elements share electrons in order to become more stable. This type of bond is characterized by the electrostatic attraction between two oppositely charged ions, which results from the transfer of electrons from one atom to the other.
The magnitude of the electrostatic force between two ions is determined by their charges, the distance separating them, and the attractive force of the nucleus of the donor atom. The ionic bond strength is also affected by the number of electrons transferred, properties such as the size of the ions, and the energy of the electronic orbitals involved.
Ionic bonds are typically formed between atoms of different electronegativities. Electronegativity is a measure of an atom’s ability to attract electrons and is determined by the number of protons in the nucleus and the number of electrons in the outer shell. When two atoms with different electronegativities interact, the atom with the higher electronegativity will attract the electrons, resulting in an ionic bond.
[toc]
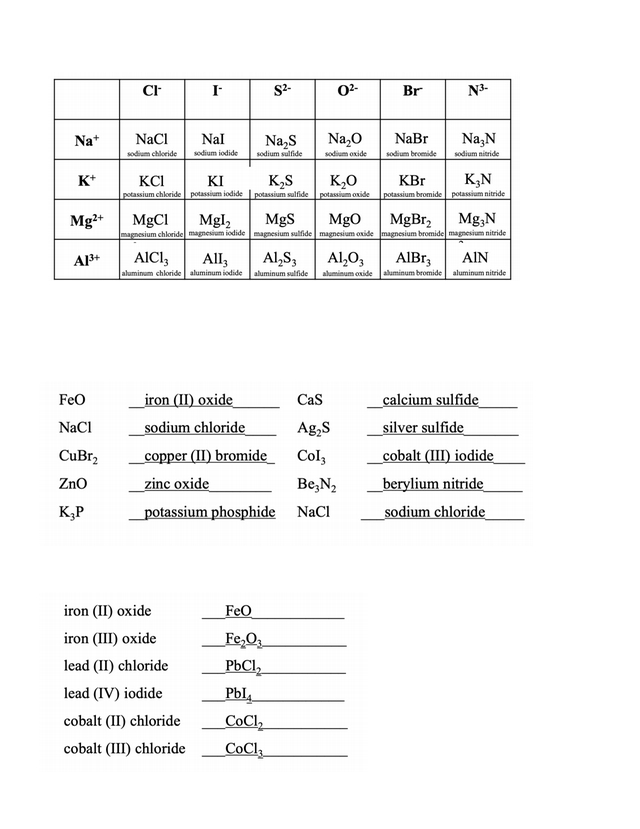
Ionic bonds are usually formed between metals and non-metals. Metals tend to lose electrons, forming cations, while non-metals tend to gain electrons, forming anions. The transfer of electrons from one atom to the other creates an electrostatic attraction between them, resulting in an ionic bond.
Ionic bonds are usually strong and usually do not break easily. They are also non-directional, meaning that the bond does not point in any particular direction. However, they can be broken apart by a process called hydrolysis, which involves the use of water as a solvent.
In conclusion, ionic bonds are formed when atoms of different elements share electrons in order to become more stable. They are characterized by the electrostatic attraction between two oppositely charged ions, which results from the transfer of electrons from one atom to the other. These bonds are typically formed between atoms of different electronegativities, usually between metals and non-metals. Ionic bonds are usually strong and non-directional, but can be broken apart by hydrolysis.
Exploring the Different Types of Chemical Bonds: Covalent, Ionic, and Metallic Bonds
Chemical bonds are the force of attraction that holds atoms and molecules together. They play a fundamental role in the structure and behavior of matter, as they are responsible for the formation of both simple and complex molecules. There are three main types of chemical bonds: covalent, ionic, and metallic bonds.
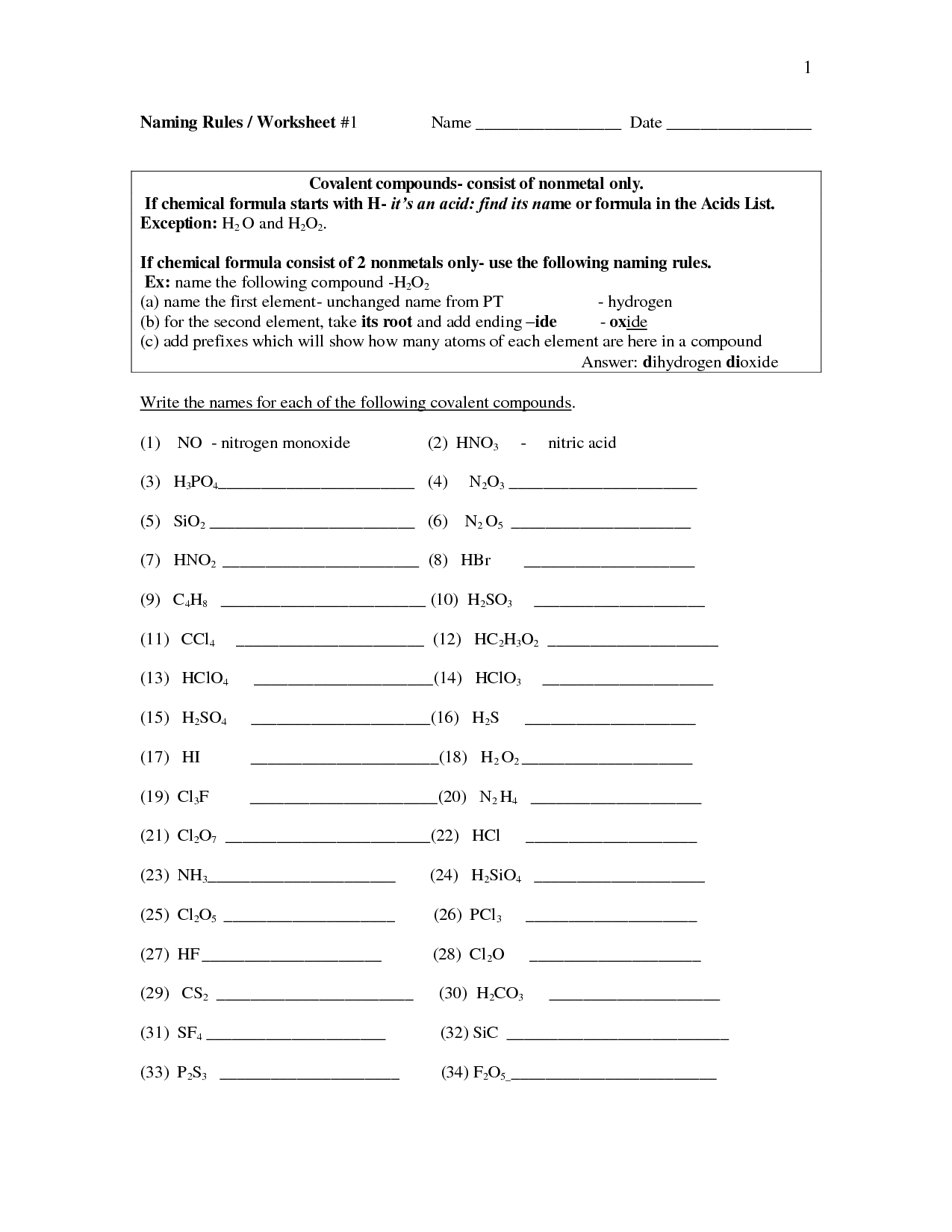
Covalent bonds are created when two atoms share electrons with each other, forming a strong bond. They are formed between two non-metals and the atoms involved form a molecule. This type of bond is characterized by its stability, as the sharing of electrons results in the atoms becoming more stable when they are together.
Ionic bonds are created when one atom donates electrons to another. This type of bonding occurs between a metal and non-metal, resulting in a positively charged metal ion and a negatively charged non-metal ion. The oppositely charged ions are attracted to each other and form a strong bond.
Metallic bonds are formed when positively charged metal ions are held together by a sea of delocalized electrons. This type of bonding forms a lattice-like structure and is characterized by its strength and flexibility. Metallic bonds are responsible for the ductility and malleability of metals, as the bonds can be easily stretched and bent.
In conclusion, covalent, ionic, and metallic bonds are the three main types of chemical bonds. Each type of bond has unique properties that make it suitable for certain applications. Understanding these bonds is essential for scientists and engineers, as they are fundamental in the formation of molecules and materials.
Investigating the Properties of Ionic Bonds: Polarity and Strength
Ionic bonds are a type of chemical bond that occurs between two ions of opposite charge. These atoms share electrons, and this electrostatic force holds them together in a lattice structure. Ionic bonds are common in compounds like sodium chloride (salt) and magnesium oxide.
Ionic bonds are polar in nature, meaning that they have a strong positive and negative charge. This is because the electrons are not shared equally between the two atoms. Instead, one atom takes on a stronger negative charge while the other takes on a stronger positive charge. This polarity is what gives ionic bonds their strength.
The strength of an ionic bond is determined by the energy it takes to break the bond. The stronger the bond, the more energy it takes to break it. The strength of an ionic bond is largely determined by the size of the ions involved. Ions that are larger in size have a stronger bond because they are able to form a stronger lattice structure.
Ionic bonds are also affected by the environment in which they form. For example, ionic bonds form more quickly in aqueous solutions because the water molecules help to stabilize the charges of the ions. In addition, if the temperature is too high, the ionic bond can become weak and break apart.
Overall, ionic bonds are strong and polar, making them an important type of chemical bond. They can form easily in aqueous solutions and their strength is determined by the size of the ions involved.
Exploring the Role of Ionic Bonds in Chemistry: From Reactions to Compounds
Ionic bonds are a type of chemical bond that is formed between two ions. These ions can either be positively charged cations or negatively charged anions. Ionic bonds are the electrostatic force of attraction between these two oppositely charged ions, which is why they are also known as electrovalent bonds. Ionic bonds are vital to the study and practice of chemistry, as they are responsible for a wide range of reactions and compounds.
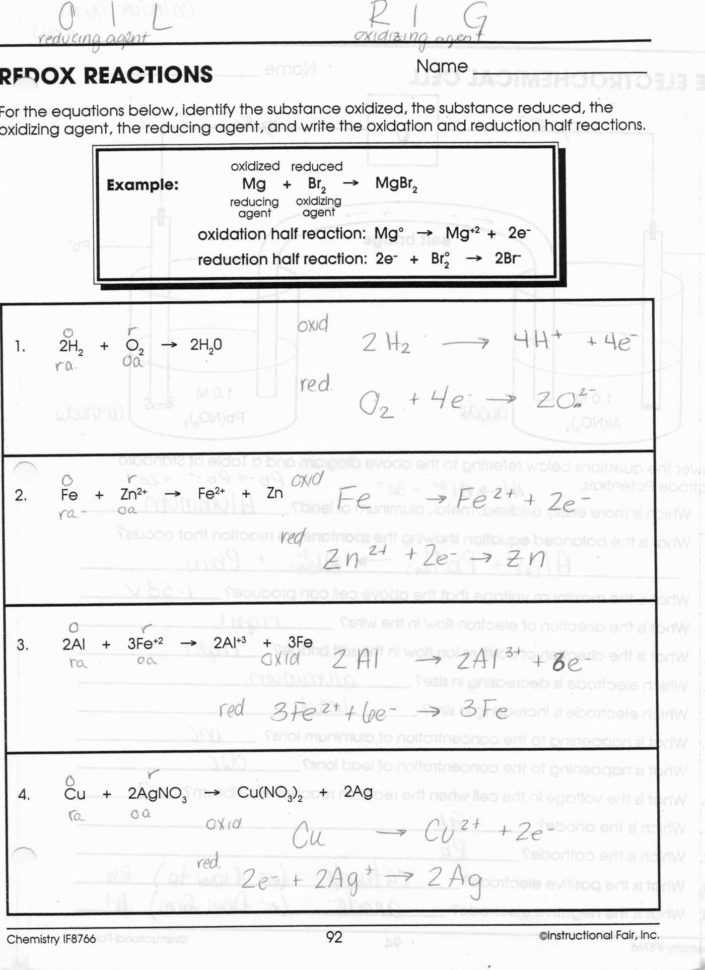
In chemical reactions, the formation of an ionic bond is a common occurrence. For example, when two molecules of hydrogen and one molecule of oxygen react, they produce a molecule of water. This reaction involves the formation of an ionic bond between the two hydrogen atoms and the oxygen atom, as the hydrogen atoms donate electrons to the oxygen atom, creating a cation and an anion. This reaction is an example of a redox reaction, which involves oxidation and reduction processes.
Ionic bonds also play a crucial role in the formation of compounds. For example, when sodium and chlorine react, they form sodium chloride (table salt). This reaction involves the transfer of an electron from the sodium atom to the chlorine atom, forming an ionic bond between them. This bond creates an electrostatic force of attraction between the two ions, which is what holds the compound together.
Ionic bonds are also important for understanding the properties of compounds. For example, because sodium chloride is held together by ionic bonds, it has a high melting point and is highly soluble in water. This is because the electrostatic force of attraction between the ions is strong enough to resist the increase in temperature and is strong enough to form a solution with water molecules.
The importance of ionic bonds in chemistry cannot be overstated. They are essential for understanding and performing a wide range of chemical reactions and compounds. From redox reactions to the properties of compounds, ionic bonds play a crucial role in understanding and using chemistry.
Conclusion
In conclusion, the Chemical Bonds Ionic Bonds Worksheet was a great tool to help us understand the basics of ionic bonds and how they work. It was an informative activity that gave us an opportunity to explore the properties of ionic bonds and how they form. By engaging with the worksheet, we were able to gain a better understanding of how ions interact with one another and how they can be used to form covalent bonds. We also learned how to recognize the different types of ionic bonds and how they can contribute to the formation of a variety of molecules.
[addtoany]