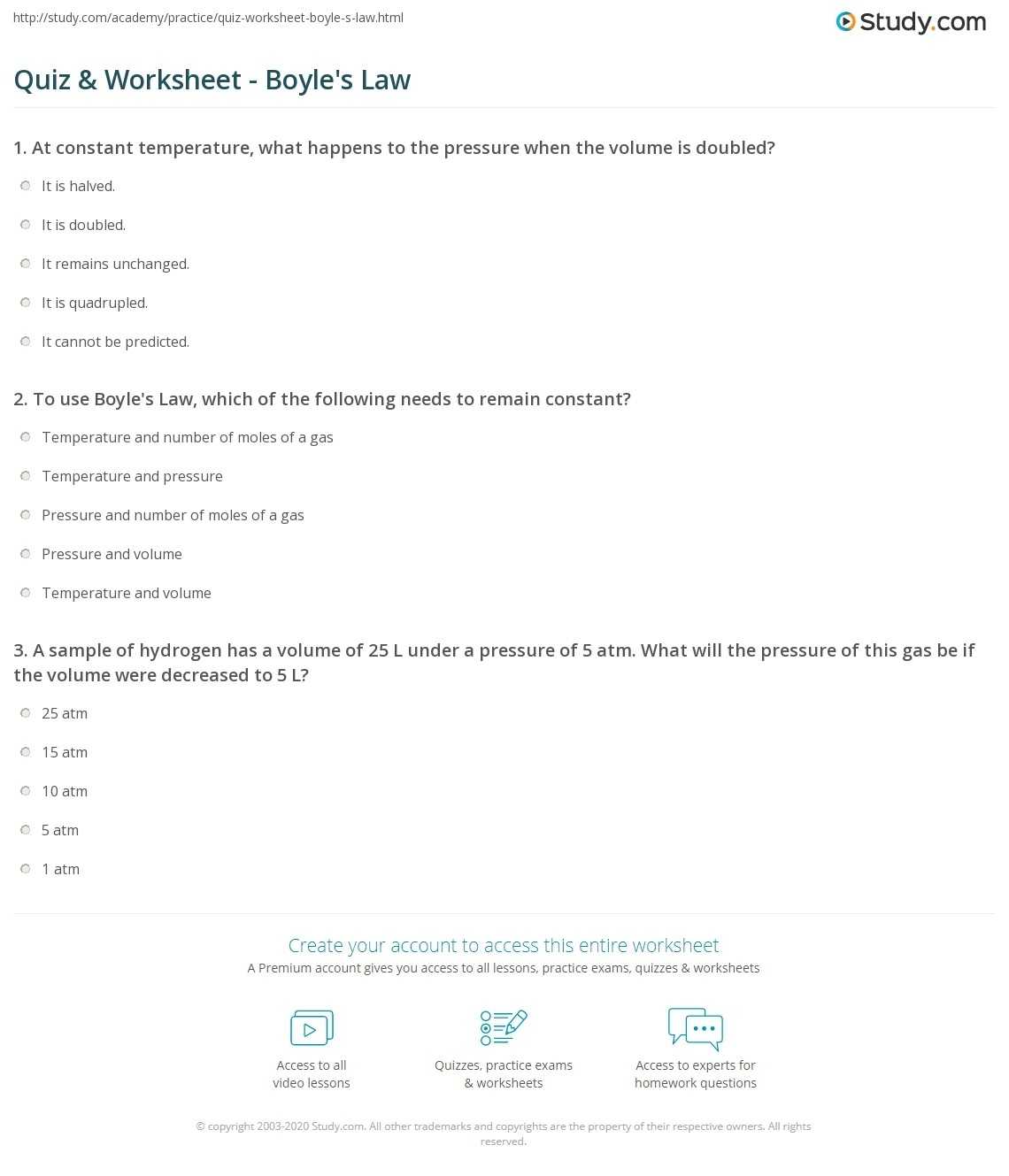
Exploring the Laws of Thermodynamics with Charles Law Worksheet Answers
The laws of thermodynamics are foundational principles underlying the study of all systems that involve heat, work, and energy. Charles Law is a fundamental thermodynamic law that states that the volume of a given mass of gas is directly proportional to its temperature, when pressure is held constant. This law has far-reaching implications in the study of the behavior of gases, and thus it is important to explore its implications in greater detail.
To begin, it is important to understand the mathematical expression of Charles Law, which is expressed as V/T = k. Here, V is the volume of the gas, T is the temperature, and k is a proportionality constant. Using this equation, we can see how the volume of a gas changes as the temperature of the gas changes. For instance, if the temperature of a gas is increased, the volume of the gas will increase as well. Similarly, if the temperature of a gas is decreased, the volume of the gas will decrease.
In addition to exploring how the volume of a gas changes as its temperature changes, Charles Law can also be used to explore how pressure affects the volume of a gas. According to Charles Law, the pressure of a given mass of gas is inversely proportional to its temperature, when volume is held constant. This means that if the pressure of a given mass of gas is increased, its temperature will decrease, and vice versa.
[toc]
Finally, Charles Law can be used to explore the behavior of gases under certain conditions. For example, it can be used to calculate the maximum pressure of a gas at a given temperature, as well as its minimum volume at a certain pressure. The implications of this law are far-reaching, and can be used to gain a better understanding of the behavior of gases.
In conclusion, Charles Law is a fundamental thermodynamic law that can be used to explore the behavior of gases under certain conditions. This law states that the volume of a given mass of gas is directly proportional to its temperature, when pressure is held constant. It can also be used to explore how pressure affects the volume of a gas, as well as to calculate the maximum pressure of a gas at a given temperature and its minimum volume at a certain pressure. By exploring the implications of Charles Law, we can gain a better understanding of the behavior of gases and the laws of thermodynamics.
Analyzing the Relationship between Pressure and Volume with Charles Law Worksheet Answers
Charles Law is a fundamental concept in thermodynamics which states that for a given quantity of an ideal gas, the volume of the gas is directly proportional to its temperature, provided that the pressure and amount of the gas remain constant. This law is also known as the Thermal Expansion Law, and it has been used to explain the behavior of gases for centuries.
In order to examine the relationship between pressure and volume, we must first understand the components of Charles Law. The equation for Charles Law is as follows: V/T = k, where V is the volume of the gas, T is the temperature, and k is a constant. This equation can be used to analyze the behavior of a gas at different temperatures and pressures.
When pressure increases, the volume of the gas decreases. This is because the molecules of the gas are now pressed closer together, which reduces the amount of space available. As the pressure increases, the temperature of the gas will also increase. This is because the molecules of the gas are now subjected to higher temperatures due to the increased pressure, which causes them to move more quickly and occupy more space.
Conversely, when the pressure decreases, the volume of the gas increases. This is because the molecules of the gas are now spread out more, which increases the amount of space available. As the pressure decreases, the temperature of the gas will also decrease. This is because the molecules of the gas are now subjected to lower temperatures due to the decrease in pressure, which causes them to move more slowly and occupy less space.
Therefore, it is evident that there is a direct relationship between pressure and volume, as outlined by Charles Law. The higher the pressure, the lower the volume, and the lower the pressure, the higher the volume. This relationship is further demonstrated by plotting pressure and volume on a graph. As pressure increases, the graph shows a decrease in volume, and vice versa.
In conclusion, Charles Law outlines the relationship between pressure and volume. As pressure increases, the volume of the gas decreases, and as pressure decreases, the volume of the gas increases. This relationship is further demonstrated by plotting pressure and volume on a graph.
Examining the Impact of Temperature Changes on Pressure and Volume with Charles Law Worksheet Answers
Charles Law states that the volume of a gas is directly proportional to its temperature, provided the pressure is kept constant. This principle can be used to examine the impact of temperature changes on pressure and volume.
When the temperature of a gas is increased, its volume will increase as well. This is due to the fact that the molecules of the gas will be moving faster as the temperature rises. This increase in the speed of the molecules will cause them to spread out in the container, resulting in an increase in volume. At the same time, the pressure exerted by the gas molecules on the walls of the container will remain constant.
On the other hand, when the temperature of a gas is decreased, its volume will decrease. This is because the molecules of the gas will be moving slower as the temperature decreases. This decrease in the speed of the molecules will cause them to move closer together in the container, resulting in a decrease in volume. Again, the pressure exerted by the gas molecules on the walls of the container will remain constant.
In conclusion, Charles Law states that the volume of a gas is directly proportional to its temperature, provided the pressure remains constant. Thus, when the temperature is increased, the volume of the gas will increase, while when the temperature is decreased, the volume of the gas will decrease. This demonstrates that temperature changes can have a significant impact on the pressure and volume of a given gas.
Conclusion
In conclusion, Charles Law Worksheet Answers provide an excellent resource for understanding the concepts of Charles’ Law, including the relationship between temperature and volume. Through the completion of this worksheet, students can gain a better understanding of the law and the implications it can have on their studies. Ultimately, by using this worksheet, students can expand their understanding of the law and how it applies to everyday life.
[addtoany]