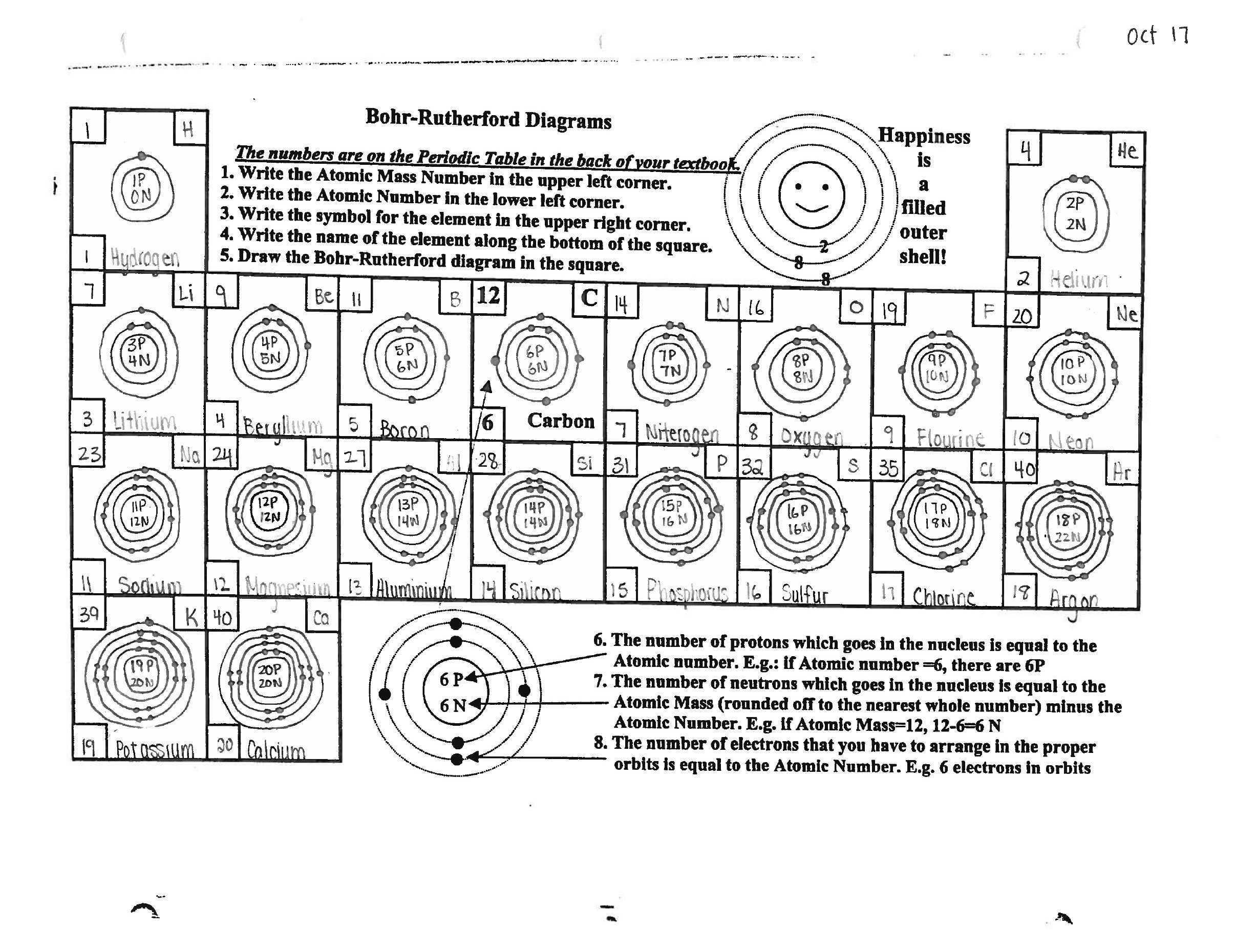
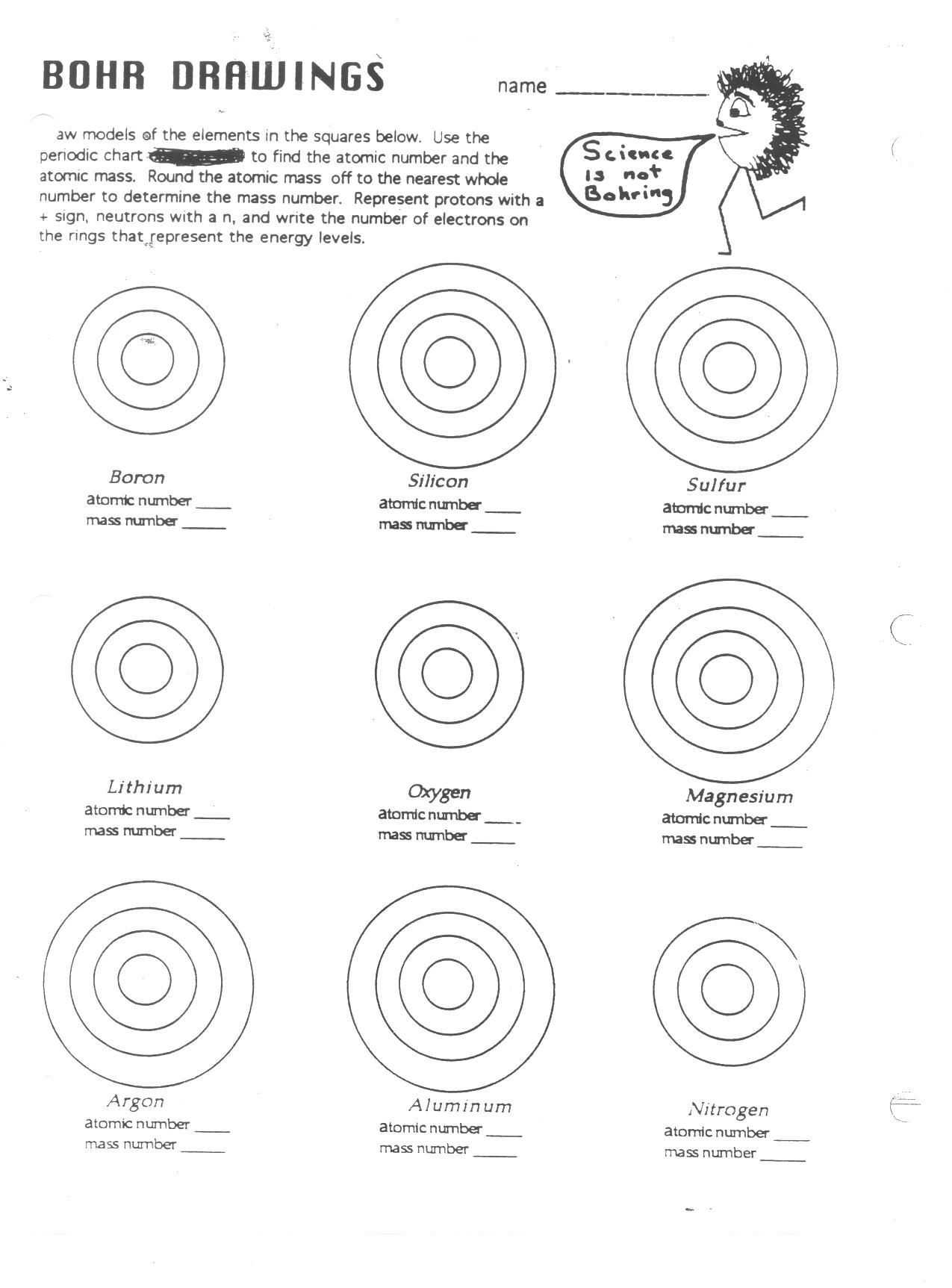
Exploring the Mysteries of the Bohr Model Worksheet Answers
The Bohr Model is a widely accepted theoretical model of the atom that was developed in the early twentieth century. Its development was a major milestone in understanding the structure and behavior of atoms. The model, proposed by Danish physicist Niels Bohr, is still used today to explain the behavior of atoms and their electrons.
The Bohr Model states that electrons are found in particular orbits around the nucleus of an atom. According to the model, each orbital has a certain energy associated with it. Electrons can only exist in orbits with specific energies, so only certain energies can be present. The energy of each orbital is determined by its distance from the nucleus. The closer an orbital is to the nucleus, the lower its energy.
The Bohr Model also explains the behavior of electrons when they gain or lose energy. When an electron gains energy, it moves to a higher-energy orbital further from the nucleus. When an electron loses energy, it moves to a lower-energy orbital closer to the nucleus. This behavior is known as the “quantum leap”.
[toc]
Despite its success, the Bohr Model has some limitations. It does not explain why electrons gain or lose energy, or how they do so. It also cannot explain the behavior of atoms with more than one electron. Finally, it cannot explain the behavior of atoms with more than one nucleus.
However, the Bohr Model still provides a useful starting point for understanding the structure and behavior of atoms. Its principles are still employed in modern physics and chemistry to explain the behavior of atoms and their electrons. The Bohr Model is an important part of our understanding of the quantum world and its mysteries.
Analyzing the Impact of the Bohr Model Worksheet Answers on Chemistry
The Bohr model of the atom has had a significant impact on the study of chemistry. Developed by Danish physicist Niels Bohr in 1913, the model provided a framework for the understanding of atomic structure and the behavior of electrons in atoms. This model was revolutionary in that it showed that electrons are not randomly distributed in an atom, but instead occupy distinct energy levels. This model also provided insight into the structure of the periodic table, which is the basis of modern chemistry.
The Bohr model of the atom has been instrumental in understanding the behavior of electrons in molecules. This has enabled chemists to predict the properties of molecules based on their structure. The model has also been used to explain the behavior of chemical reactions, as well as the stability of molecules. Additionally, the model has allowed chemists to understand the structure and properties of organic molecules, which are the building blocks of all living things.
The Bohr model of the atom has also provided insight into the behavior of light. This model has improved our understanding of the behavior of photons, which are particles of light, and the mechanisms of light absorption and emission. This has enabled chemists to create lasers and other light-based technologies.
The Bohr model of the atom has been an invaluable tool in the study of chemistry. It has revolutionized our understanding of atomic structure and the behavior of electrons in atoms. This model has enabled chemists to better predict the properties of molecules and the behavior of chemical reactions. It has also improved our understanding of light and its properties, which has enabled chemists to create lasers and other light-based technologies. The Bohr model of the atom continues to be an invaluable tool in the study of chemistry and its many applications.
How the Bohr Model Worksheet Answers Enhance Teaching and Learning
The Bohr Model worksheet answers are an invaluable teaching and learning tool for educators and students. By providing detailed explanations of the various concepts, the worksheet answers allow educators to provide an in-depth, comprehensive understanding of the material. This helps to ensure that students retain the material and are better equipped to apply it in their everyday lives.
The worksheet answers also allow for the exploration of many different topics related to the Bohr Model. This allows for a deeper level of understanding as students can investigate different concepts and apply them to their own situations. This allows for an interactive learning experience and encourages students to think critically and creatively about the material.
The worksheet answers provide students with the opportunity to practice their problem-solving skills. As they complete the worksheet, they can identify patterns, draw conclusions, and apply their knowledge in real-world scenarios. This allows them to develop their critical thinking skills, which is an important part of any successful academic career.
The worksheet answers also provide an opportunity to engage in meaningful conversations with their peers. As students complete the worksheet, they can discuss their findings and share their insights with their classmates. This encourages collaboration and increases the likelihood of meaningful conversations about the material.
Overall, the Bohr Model worksheet answers provide an effective way to teach and learn about the Bohr Model. By providing detailed explanations and allowing for meaningful conversations, the worksheet answers help to ensure that students retain the material and are better equipped to apply it in their everyday lives.
Comparing Different Approaches to Solving the Bohr Model Worksheet Answers
The Bohr model worksheet is a classic problem in quantum mechanics. It involves solving for the energy levels of hydrogen atoms in their ground state. There are several different approaches to solving this problem, each of which can yield an accurate result.
The most common approach is to solve the Schrödinger equation for a single electron in a central potential. This involves solving a set of differential equations and finding the energy eigenvalues of the system. This approach is both accurate and reliable, but can be computationally intensive.
Another approach is to use the WKB approximation. This is a semi-classical approximation which can be used to approximate the energy levels of a system. The WKB approximation is relatively straightforward to implement, but can result in inaccuracies in certain cases.
The third approach is to use the Heisenberg Uncertainty Principle. This approach relies on the fact that the energy of a system is uncertain due to the interaction between the particles. This uncertainty can be used to approximate the energy levels of a system.
Finally, there is the Hartree-Fock method. This is an ab initio method which uses the correlation of electrons to calculate the total energy of a system. This approach is more accurate than the WKB approximation and can be used to calculate the energy levels of a system with greater precision.
Each of these approaches has its own advantages and disadvantages. Depending on the specific problem, one approach may be more suitable than another. However, all of these approaches can be used to accurately solve the Bohr model worksheet.
Conclusion
The Bohr Model Worksheet Answers provide a great insight into the atomic structure of an atom. It is a great way to understand how the electrons and protons interact with one another to form the structure of an atom. Through the worksheet, we are able to see how the Bohr Model works and how this model is used to explain the behavior of an atom. Understanding the Bohr Model is essential for anyone studying chemistry or physics.
[addtoany]