The Benefits of Understanding Basic Atomic Structure: A Look at a Basic Atomic Structure Worksheet Answer Key
Atoms are the building blocks of life and understanding their basic structures is essential for anyone studying chemistry or biology. By understanding the basics of atomic structure, students can gain a better grasp of the properties of matter and how chemical reactions occur. An understanding of basic atomic structure can also be used to explain physical phenomena, such as the behavior of light, electrical conductivity, and the motion of subatomic particles.
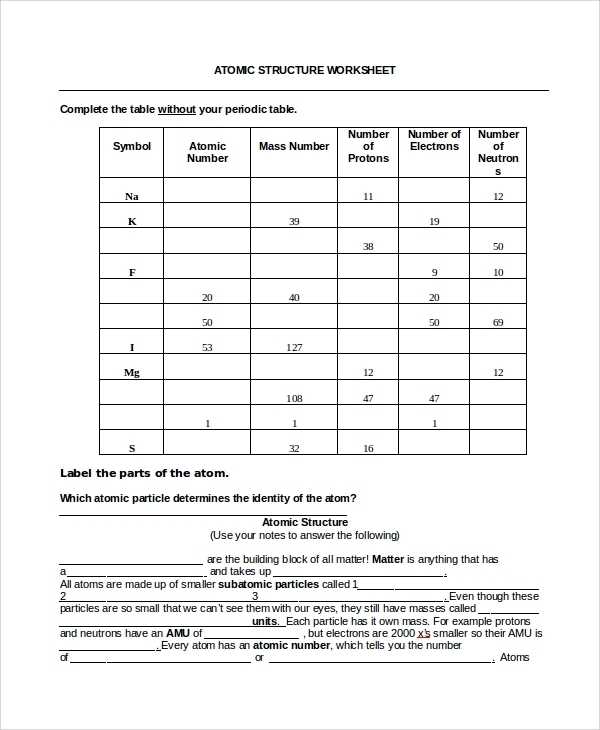
A basic atomic structure worksheet answer key can provide students with an efficient way to learn the basics of atomic structure. A worksheet answer key provides students with a comprehensive overview of the various components of an atom, including its nucleus, electrons, and protons. Students can use the answer key to quickly identify and label each part of an atom and its subatomic particles.
By working through the worksheet answer key, students can gain a better understanding of the different components of an atom and how they interact with each other. They can also learn about the various types of elements and compounds and how they interact with one another. With an understanding of basic atomic structure, students can develop an understanding of the many properties of matter and their effects on the environment.
[toc]
In addition to gaining an understanding of basic atomic structure, a basic atomic structure worksheet answer key can also provide students with an understanding of how chemical reactions occur. Students can learn about the different types of bonds between atoms and the different types of reactions that can take place. This understanding can help students understand how matter works and how changes in its composition can affect the environment.
Finally, understanding basic atomic structure can help students understand the different properties of elements and how they interact with one another. By learning about the different properties of elements, students can develop an understanding of how various substances interact with each other and how they can be used to create new substances. This understanding can help students understand the various properties of substances and how they can be used to create new materials or products.
Exploring Atoms and Molecules with a Basic Atomic Structure Worksheet: Tips for Success
1. Read up on the topic: The best way to understand atoms and molecules is to learn about them from reliable sources. Before you get started on the worksheet, take some time to read up on the basics of atomic structure. This will help to ensure that you understand the concepts as you work through the questions.
2. Make notes: It is important to pay attention to the details as you work through the worksheet. Make sure to make notes as you go along so that you can refer back to them later on. This will help to ensure that you are able to answer the questions accurately.
3. Double check your answers: As you work through the worksheet, make sure to double check your answers. This will help to make sure that you are giving accurate answers and not just guessing.
4. Ask for help: If you find yourself struggling with certain concepts or questions, don’t hesitate to ask for help. Talk to your teacher, a tutor, or a mentor for assistance.
5. Take your time: Don’t rush through the worksheet. Take your time and focus on understanding the concepts. This will help you to answer the questions correctly.
6. Review your work: Once you are finished with the worksheet, take some time to review your answers. This will help you to identify any mistakes that you may have made and give you the opportunity to correct them.
Uncovering the Mysteries of the Atom: An Overview of Basic Atomic Structure Worksheet Answers
Atomic structure is a fascinating and complex topic, with a deep history of scientific research and exploration. It is an essential part of understanding the basic components of matter and the universe as a whole. This worksheet provides an overview of the structure of the atom, its components, and its behavior.
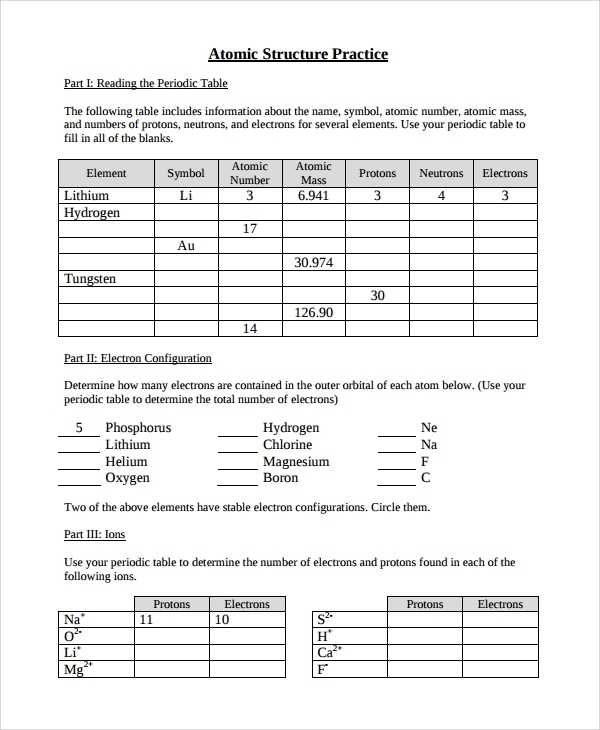
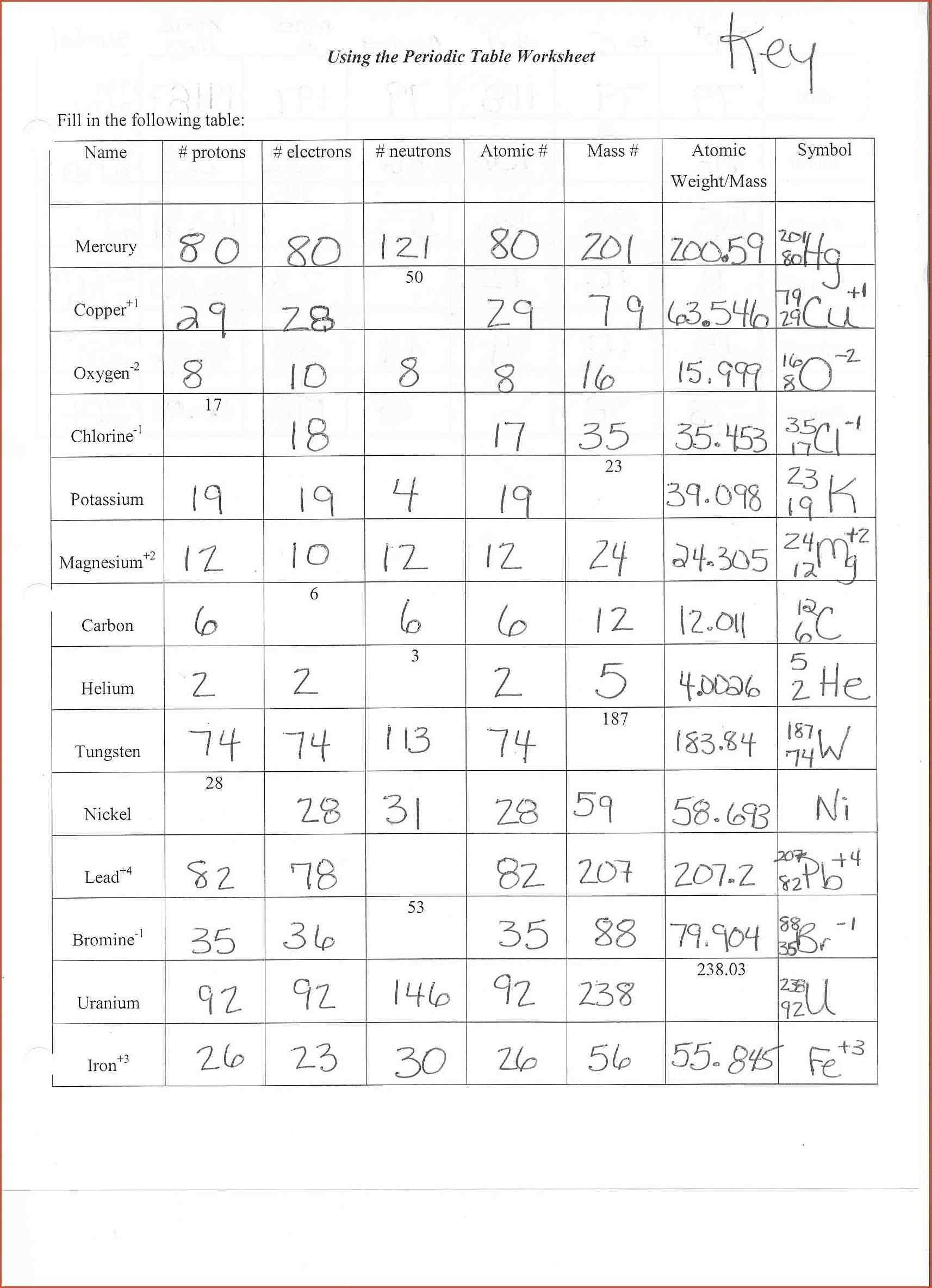
The atom is the smallest unit of matter that can exist in its elemental form. It is composed of three main components: protons, neutrons, and electrons. Protons are positively charged particles located in the nucleus of the atom and are responsible for most of its mass. Neutrons are found in the nucleus and are neutral particles with no charge. Electrons are particles with a negative charge and are located in the outer shell of the atom.
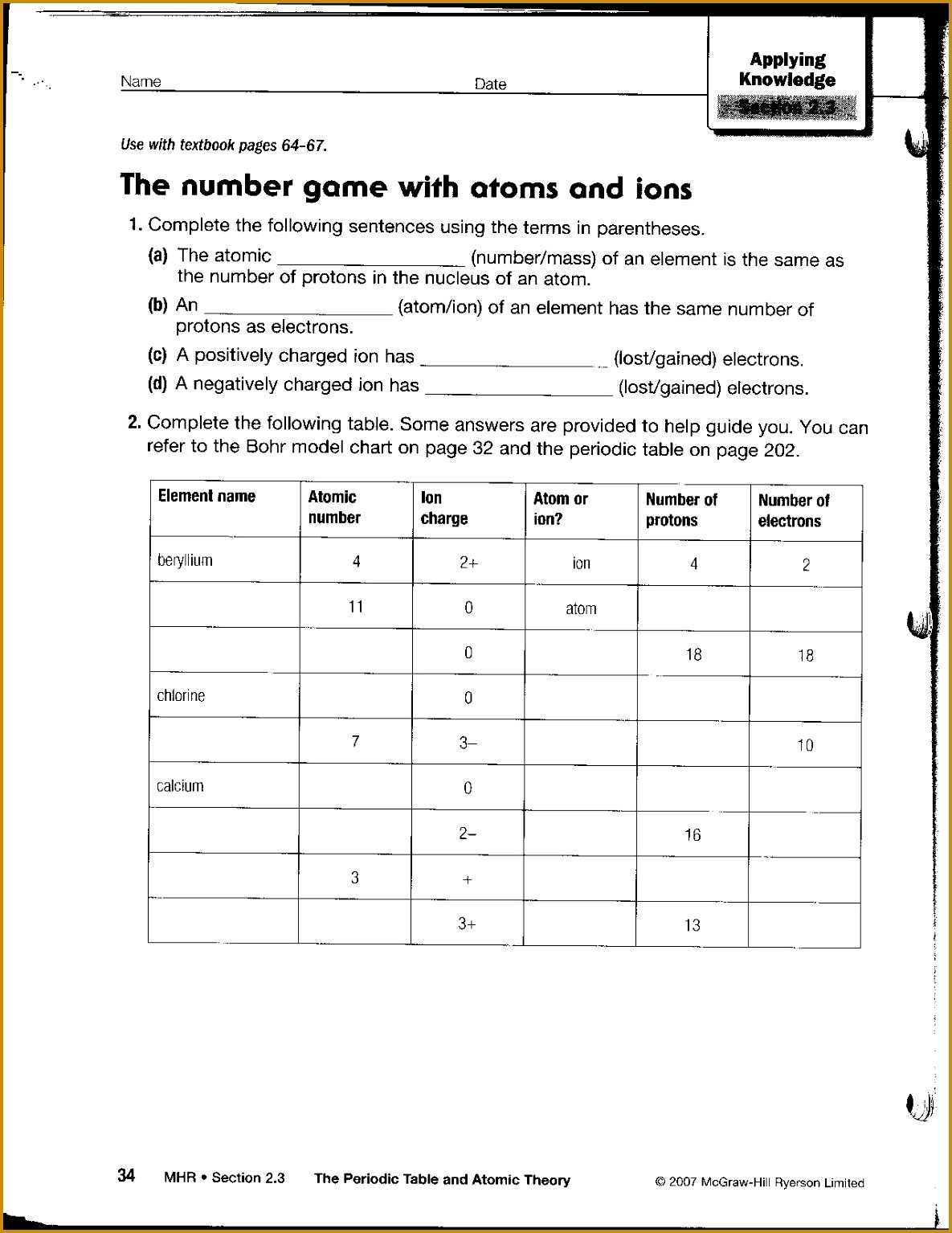
The number of protons in an atom determines its identity as a particular element. This number is known as the atomic number and is specific to each element. For example, hydrogen has an atomic number of 1, while oxygen has an atomic number of 8. The number of neutrons in an atom can vary, but it is usually close to the number of protons.
The behavior of an atom is determined by the number of electrons in its outer shell. Atoms can interact with one another when they have a different number of electrons. Atoms with more electrons than protons are negatively charged, while atoms with fewer electrons than protons are positively charged. Atoms with the same number of electrons, protons, and neutrons are said to be neutral and do not interact with other atoms.
Atoms can combine to form molecules, which are groups of atoms joined together. Molecules are extremely important in chemistry, as different molecules interact with one another in different ways. For example, water is composed of two hydrogen atoms and one oxygen atom and is essential for life on earth.
This worksheet has provided a basic overview of atomic structure and its components. It has highlighted the importance of protons, neutrons, and electrons in determining the properties and behavior of an atom, as well as how atoms can combine to form molecules. Understanding atomic structure is essential for furthering our knowledge of the universe and the building blocks of matter.
Investigating the Building Blocks of Matter: A Guide to Basic Atomic Structure Worksheet Answers
The structure of matter is one of the most fundamental concepts in science. At its core, matter is composed of atoms, the building blocks of all physical things. Understanding the basic atomic structure is essential for a thorough understanding of matter and its behavior. This guide provides an overview of the components of an atom and the interactions between them.
At its most basic level, an atom is composed of protons, neutrons, and electrons. Protons have a positive charge and neutrons have no charge; these two particles form the nucleus of an atom. The negatively charged electrons orbit around the nucleus, forming a “shell” of energy around the nucleus. The number of protons and electrons in an atom determines its atomic number and the identity of the element.
The electrons in an atom are arranged in specific energy levels, or shells. Each shell can contain a certain maximum number of electrons. As electrons are added to a shell, they become less tightly bound to the nucleus, and as they move further away from the nucleus, they become more loosely bound. As a result, the shells of an atom can be thought of as concentric circles, each with a certain maximum capacity.
The arrangement of the electrons in an atom is determined by the laws of quantum mechanics. These laws state that the electrons can only occupy certain energies, or “orbitals”, around the nucleus and that they must fill the orbitals in a particular order. This order is known as the “Aufbau principle” and dictates that electrons fill the lowest energy orbitals first before moving to higher energy orbitals.
The electrons in an atom also experience forces of attraction and repulsion. Electrons in the same shell experience a force of repulsion, while electrons in different shells experience a force of attraction. This force of attraction is known as the “Coulomb force” and helps to keep the electrons in their orbitals.
Finally, the protons and neutrons in the nucleus of an atom also experience forces of attraction and repulsion. This is known as the “strong nuclear force” and is responsible for keeping the nucleus together.
In summary, atoms are composed of protons, neutrons, and electrons which are arranged in shells of energy around the nucleus. The arrangement of the electrons is determined by the laws of quantum mechanics, and the forces of attraction and repulsion between the protons, neutrons, and electrons help to keep the atom together. Understanding the basic atomic structure is essential for understanding the behavior of matter.
Conclusion
Overall, the Basic Atomic Structure Worksheet Answers provide a great foundation for understanding the basics of atomic structure and its components. The worksheet is a great tool for students to learn about the atom and its components, as well as the different types of particles that make up the atom. It is important for students to be able to understand the basics of atomic structure in order to be successful in their studies of the physical world.
[addtoany]