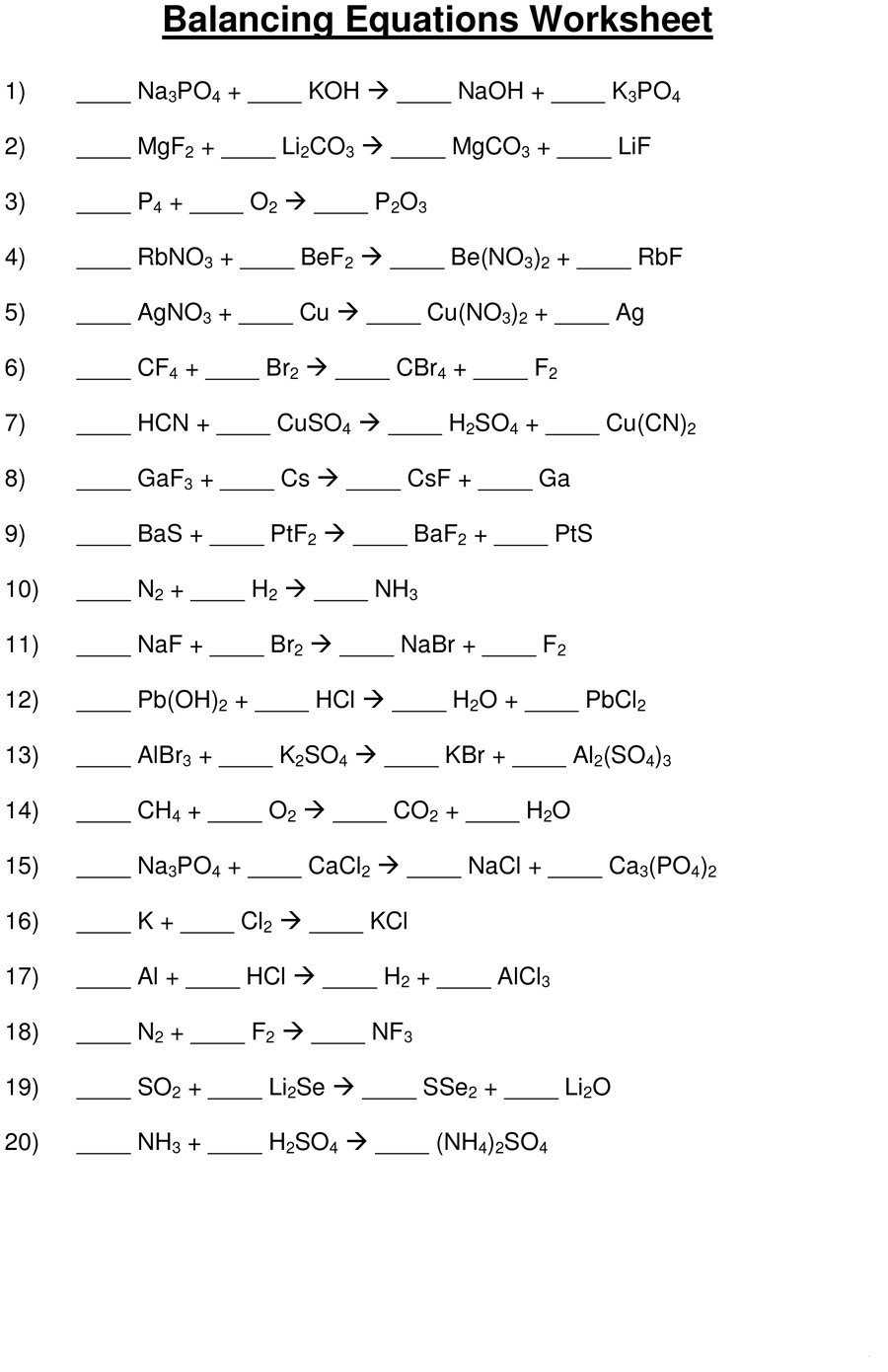
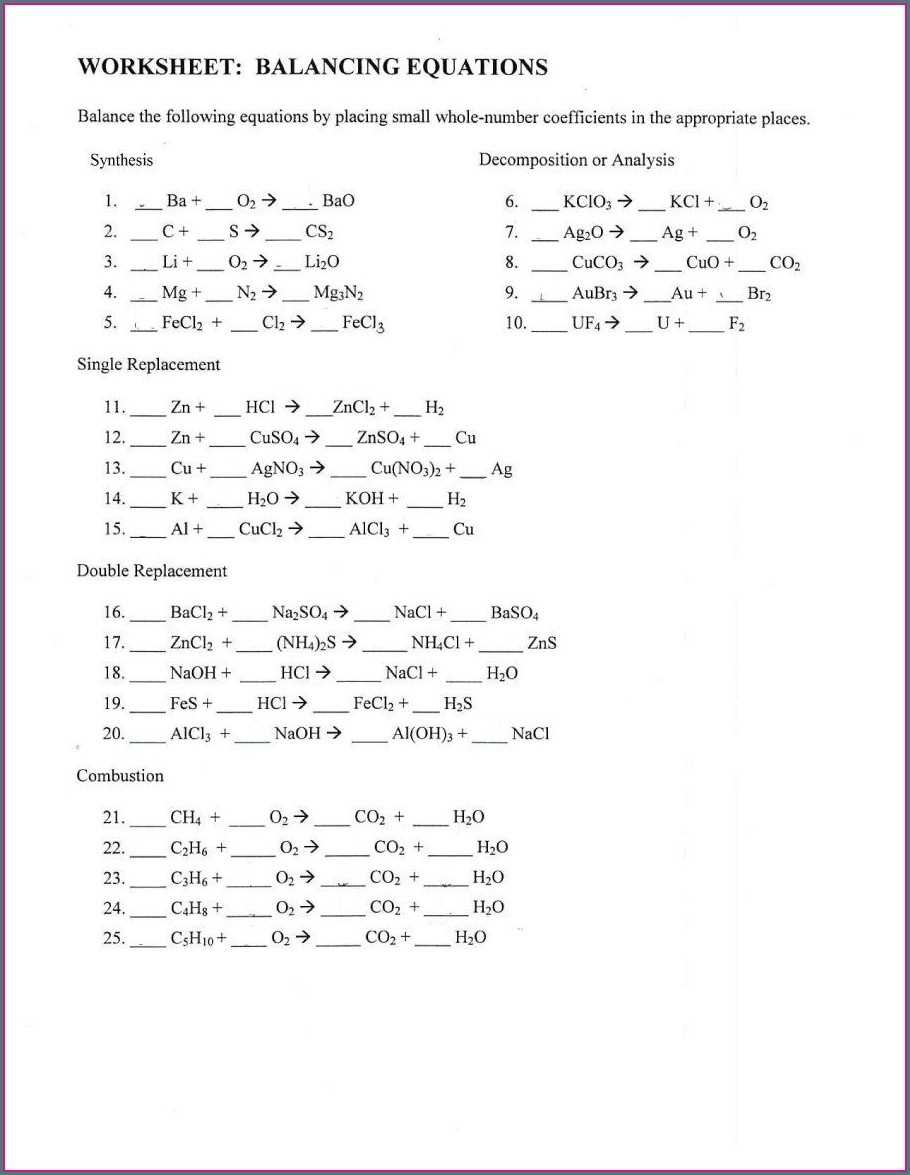
How to Use a Balancing Equations Worksheet Answer Key to Test Your Understanding of Chemical Reactions
Using a balancing equations worksheet answer key can be an effective way to assess your understanding of chemical reactions. The answer key can provide the correct answer to each reaction and allow you to assess if your own answer is correct or incorrect.
When using a balancing equations worksheet answer key, it is important to take your time and carefully review each answer provided. Carefully analyze the reaction, and make sure that the number of atoms on both sides of the equation are equal. Also, make sure that the correct types of atoms are present on each side of the equation. Additionally, it is important to pay close attention to the state of the reactants and products; for example, the reactants should be written as the reactants in their elemental form, not their compounds.
Once you have carefully reviewed each answer, you can check your answer against the answer key. If you find that your answer is different from the one provided, take your time to identify any mistakes that you may have made and make the necessary corrections.
[toc]
By taking the time to use a balancing equations worksheet answer key, you can ensure that your understanding of chemical reactions is accurate and that you are able to properly balance equations.
Using Balancing Equations Worksheet Answer Keys to Master Chemical Equations
Balancing equations worksheets are an invaluable tool for students to master the complex process of balancing chemical equations. By using answer keys with these worksheets, students can quickly and accurately check their work for accuracy.
Answer keys provide the correct answer to each equation, allowing students to quickly verify their answers. This helps them to identify where they may have made a mistake and correct it. Additionally, answer keys provide the chemical formula or equation of each reaction, helping students to understand the chemical reaction process.
Balancing equations worksheets also provide students with practice in understanding the concept of conservation of mass. As they work through the equations, they are able to observe and identify which elements are conserved and which are changed in a given reaction. This encourages a deeper understanding and retention of the material.
Answer keys also provide an opportunity for students to practice the process of solving equations. By using answer keys, students can quickly become familiar with the process of balancing equations and then apply the same techniques to more complex equations.
By using answer keys, students can also gain an appreciation of the importance of following chemical safety rules and procedures. Many worksheets provide safety reminders and warnings, which can be reinforced when using answer keys.
By using answer keys with balancing equations worksheets, students are better equipped to master the concepts of chemical equations. With the help of answer keys, students can quickly and accurately check their work to ensure accuracy and safety. This allows them to develop a deeper understanding of the material and apply their knowledge to more complex equations.
Understanding the Basics of Balancing Equations: A Step-by-Step Guide with Worksheet Answer Keys
Balancing equations is a fundamental skill in chemistry, as it allows chemists to determine the actual number of atoms that are participating in a chemical reaction. In this step-by-step guide, we will explore the basics of balancing equations, including the rules governing the process and a practice worksheet with answer keys.
The Rules of Balancing Equations
The process of balancing chemical equations requires the use of certain rules that govern the process. These rules must be followed in order to successfully balance an equation.
Rule 1: The number of atoms for each element must be the same on both sides of the equation.
Rule 2: The total charge of the reactants must be equal to the total charge of the products.
Rule 3: The total number of atoms of each element must be the same on both sides of the equation.
Rule 4: The equation must be balanced in terms of mass, meaning that the total mass of the reactants must be equal to the total mass of the products.
Practice Worksheet
Now that we have reviewed the rules of balancing equations, let us try our hand at balancing some chemical equations. The following worksheet consists of several equations that need to be balanced.
1. CH4 + O2 → CO2 + H2O
2. K2O + H2 → KOH + H2O
3. C6H12O6 + O2 → CO2 + H2O
4. C2H6 + O2 → CO2 + H2O
Answer Keys
1. CH4 + 2O2 → CO2 + 2H2O
2. K2O + 2H2 → 2KOH + H2O
3. C6H12O6 + 6O2 → 6CO2 + 6H2O
4. 2C2H6 + 7O2 → 4CO2 + 6H2O
Conclusion
The Balancing Equations Worksheet Answer Key is an invaluable resource for students who are learning how to balance equations. It provides clear and detailed explanations for each equation, as well as a variety of techniques and strategies to help students understand the concept of balancing equations. By practicing with the worksheet and answer key, students can gain a better understanding of the material and improve their skills in balancing equations.
[addtoany]