Exploring the Benefits of Using an Average Atomic Mass Worksheet to Improve Chemistry Grades
The average atomic mass worksheet is a valuable tool in the study of chemistry. It is designed to help students understand the concept of average atomic mass by providing them with an easy-to-follow worksheet to guide them through the process. By using an average atomic mass worksheet, students can gain a better understanding of the topic, improve their chemistry grades, and increase their confidence in the subject.
The average atomic mass worksheet helps to bridge the gap between the theoretical and practical aspects of chemistry. By providing students with a worksheet to help them calculate the average atomic mass of elements, they can gain a better understanding of the concept. This worksheet allows students to practice and apply their knowledge of the topic in a practical way. Furthermore, it allows them to make mistakes and learn from them, thereby improving their understanding and confidence.
The average atomic mass worksheet also helps to build upon students’ existing knowledge of the subject. By providing them with a comprehensive overview of the topic, students can gain a better understanding of the concept. It also helps to reinforce their understanding of the material by providing them with a step-by-step guide to the process.
[toc]
The average atomic mass worksheet also provides students with an opportunity to practice their problem-solving skills. By working through the worksheet, students can gain a greater appreciation for the math and science involved in the topic. This helps them to become more confident in their ability to solve problems and increase their chances of achieving a higher grade in their chemistry class.
The average atomic mass worksheet also provides students with an opportunity to work with real-life examples. By providing them with examples of the different elements, students can gain a better understanding of the concept. Moreover, it provides them with an opportunity to practice their math and science skills in a more concrete way.
The average atomic mass worksheet can be used by students of all grade levels. By providing students with a comprehensive overview of the topic, it can be used to supplement their current course material and help them gain a deeper understanding of the subject. Furthermore, it can be used to help students prepare for exams and improve their overall chemistry grades.
A Comprehensive Guide to Understanding and Interpreting Average Atomic Mass Worksheet Answers
Introduction
The average atomic mass (AAM) of an element is a measure of its mass relative to 1/12 of the mass of a carbon-12 atom. It is an important concept in chemistry, and it is essential to understanding a wide variety of topics, including the properties of matter, the structure of molecules, and the behavior of chemical reactions. This worksheet provides a comprehensive guide to understanding and interpreting AAM.
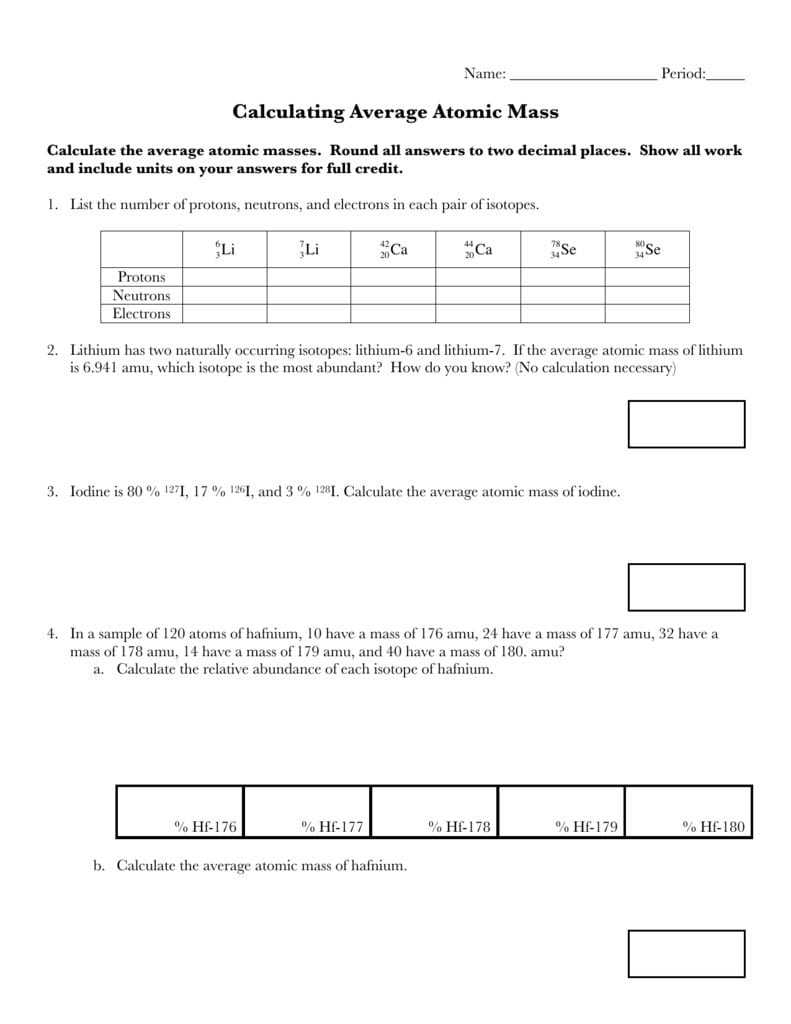
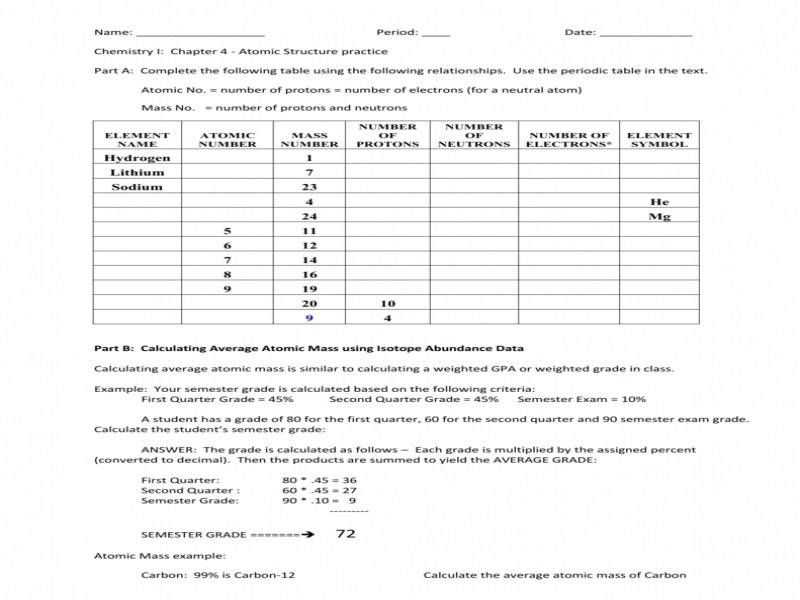
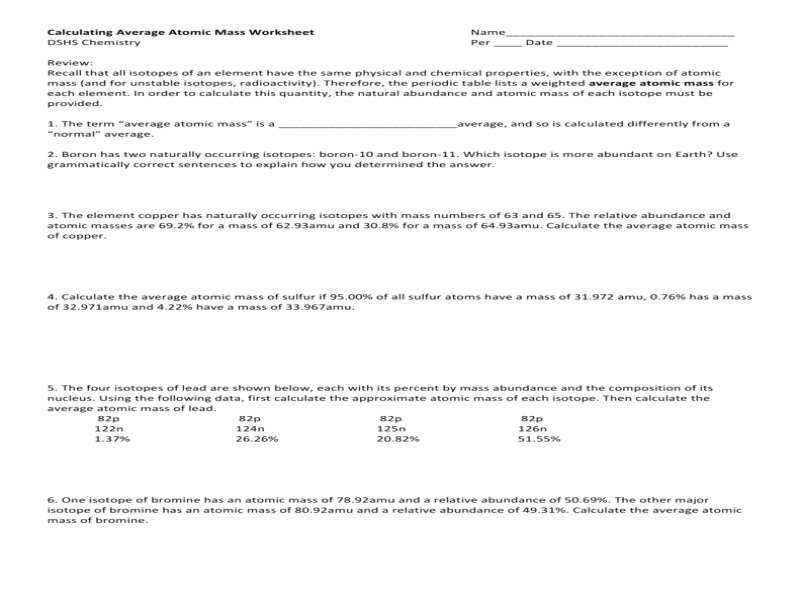
Part 1: Calculating Average Atomic Mass
AAM is calculated by taking the weighted average of the masses of all the naturally occurring isotopes of an element. To calculate the AAM, it is necessary to first determine the atomic masses of all the isotopes of the element. These can be found in any periodic table. Then, the natural abundance of each isotope must be found. This value is expressed as a percentage, and it corresponds to the proportion of atoms of that isotope which are found in nature. Finally, the atomic mass of each isotope is multiplied by its natural abundance, and the results are added together to find the AAM.
Part 2: Interpreting Average Atomic Mass
AAM can be used to compare the relative masses of different elements. For example, a comparison of the AAM of carbon (12.0107 g/mol) and oxygen (15.9994 g/mol) demonstrates that oxygen is approximately 32% heavier than carbon. AAM can also be used to compare the masses of different isotopes of the same element. For example, the AAM of chlorine (35.4527 g/mol) is composed of approximately 75.5% chlorine-35 and 24.5% chlorine-37. This indicates that chlorine-37 is heavier than chlorine-35 by a ratio of approximately 3:2.
Conclusion
Understanding AAM is essential for anyone interested in chemistry. It can be used to compare the relative masses of different elements and isotopes, and it is an important concept for understanding the structure of molecules and the behavior of chemical reactions. This worksheet has provided a comprehensive guide to understanding and interpreting AAM, including instructions for calculating AAM and interpreting the results.
Examining How to Leverage Average Atomic Mass Worksheet Answers to Enhance Chemistry Learning
The average atomic mass worksheet is a powerful educational tool for students studying chemistry. By leveraging this worksheet, students can gain a better understanding of how elements are composed and the role that atomic mass plays in the formation of molecules.
The average atomic mass worksheet is a collection of questions and diagrams that serve to help students understand the relationship between elements and the atomic masses associated with them. Students are asked to identify the element and then determine the average atomic mass of that element. By answering these questions, students gain a better understanding of how elements interact and how the atomic mass is a factor in the formation of molecules.
In addition to the questions and diagrams provided in the worksheet, teachers can use the worksheet to enhance the learning of their students by providing additional activities and experiments. For example, teachers can have students explore the periodic table of elements and make predictions about the average atomic mass of various elements. This activity gives students a better understanding of how elements interact, as well as how to make informed decisions about the atomic mass of various elements.
Another activity teachers can use to enhance the learning experience is to have students conduct experiments to determine the average atomic mass of various elements. This activity gives students the opportunity to practice their knowledge and learn more about the relationship between elements and the atomic masses associated with them.
By leveraging the average atomic mass worksheet, teachers can provide students with a better understanding of elements and the atomic masses associated with them. Through activities, experiments, and questions, students can gain a better understanding of how elements interact and how the atomic mass plays a role in the formation of molecules. This knowledge can be used to make informed decisions about the composition of molecules, which is a critical component of understanding chemistry.
An Analysis of How Average Atomic Mass Worksheet Answers can Help Students Master Chemistry Concepts
Understanding average atomic mass is an important part of mastering chemistry concepts. Utilizing an Average Atomic Mass Worksheet Answers can be an effective way for students to increase their understanding and application of this concept.
The average atomic mass worksheet provides a comprehensive overview of how to calculate the average atomic mass of an element. It begins by providing information on the atomic mass unit and how it can be used to measure the mass of an atom. It then explains the concept of relative atomic mass and the use of isotopes in calculating the average atomic mass of an element. Finally, it provides examples of how to use the calculation to determine the average atomic mass of an element.
By using the average atomic mass worksheet, students are able to gain a better understanding of the concept and how to calculate the average atomic mass of an element. Through this worksheet, they can learn the various components that go into the calculation and how to apply the formula to determine the average atomic mass of an element. They can also gain a better understanding of isotopes and how their mass affects the average atomic mass of an element.
The average atomic mass worksheet also provides students with an opportunity to practice applying the formula to different elements. By doing so, they can gain a deeper understanding of the concept and how it is used to determine the average atomic mass of an element. It can also be helpful to have students complete the worksheet with a partner, as this can provide an opportunity to discuss the concept more in depth and gain a better understanding of the material.
Overall, using an average atomic mass worksheet answers can be a great way for students to gain a better understanding of the concept and how to apply it to their studies. Through the worksheet, students can gain a better understanding of the components of the calculation and how to use it to determine the average atomic mass of an element. They can also gain a better understanding of isotopes and how they affect the average atomic mass of an element. Through practice and discussion, students can become more proficient in their understanding and application of this concept.
Conclusion
The Average Atomic Mass Worksheet Answers provides an excellent resource for students and researchers alike to gain a better understanding of the fundamentals of atomic structure and mass. It helps to further our knowledge of the basic principles of science and can be used as a basis for further research. The answers on the worksheet can serve as a useful guide to students as they explore the different aspects of atomic structure and mass.
[addtoany]