Utilizing an Average Atomic Mass Worksheet to Enhance Student Understanding of Atomic Structure
One of the most significant aspects of chemistry is atomic structure. To help students comprehend the complex nature of atoms, educators can use an average atomic mass worksheet. This worksheet provides an interactive way to learn about the composition of atoms, helping students gain a better understanding of the topic.
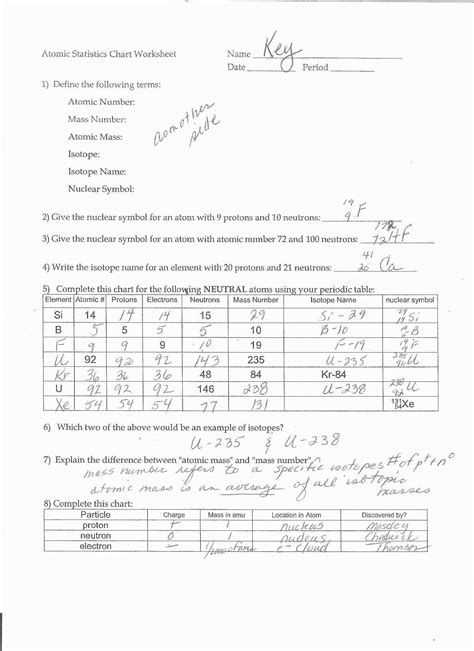
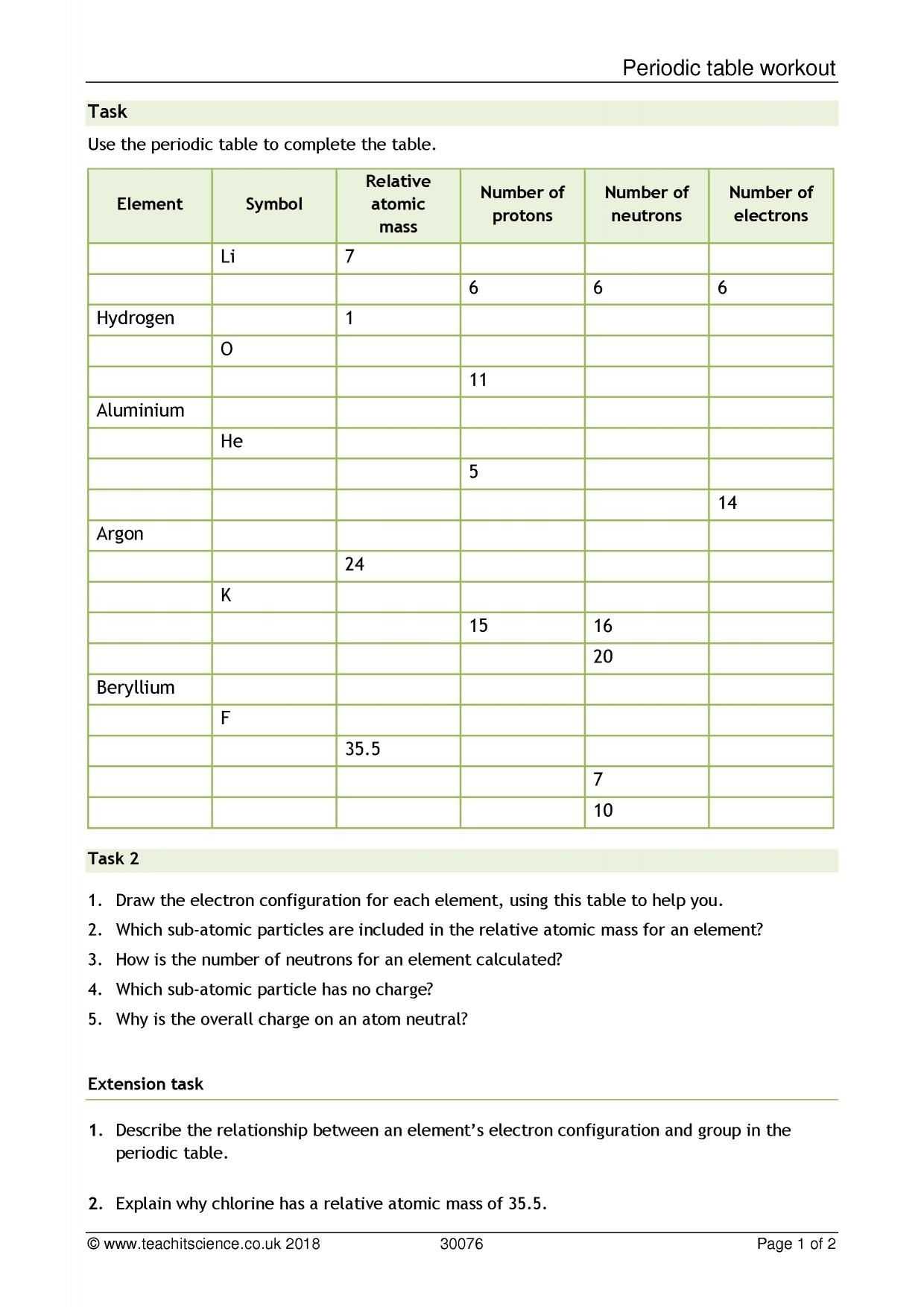
The average atomic mass worksheet typically begins with a brief explanation of atomic structure. This part of the worksheet explains how atoms are broken down into protons, neutrons, and electrons. It also explains how the protons and neutrons combine to form the nucleus of the atom.
The next step is for students to calculate the average atomic mass. To do this, they must first determine the number of protons and neutrons in each atom. Then, they can use the atomic mass unit (amu) to calculate the average atomic mass.
[toc]
Once students have calculated the average atomic mass, they can use this figure to compare the masses of different atoms. This comparison can help them visualize the different atoms and their mass relative to one another.
At the end of the worksheet, students can also explore the concept of isotopes. Isotopes are atoms that have different numbers of neutrons, but the same number of protons. This helps students understand the concept of isotopes and how they influence the atomic mass of an atom.
Overall, an average atomic mass worksheet can be a valuable tool to help students learn about the structure of atoms. By providing an interactive way to understand the topic, students gain a better understanding of the concept and can apply this knowledge when studying other topics in chemistry.
Exploring the Concept of Average Atomic Mass Through Activities on a Worksheet
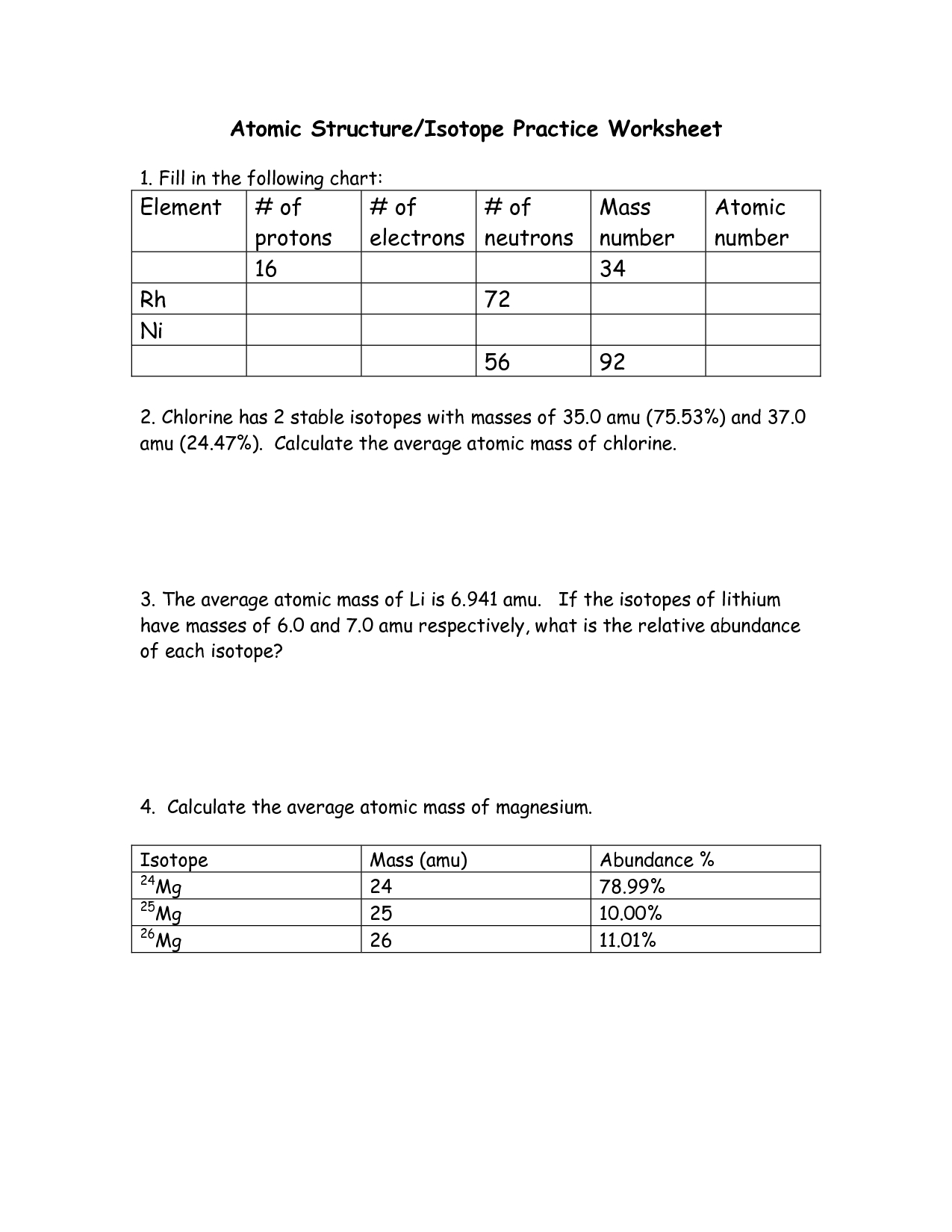
The average atomic mass of an element is a fundamental concept in chemistry, and exploring this concept through activities on a worksheet can be a great way to understand it. To begin, students should be given a worksheet with a table of the atomic masses of different elements, as well as a definition of the average atomic mass.
Next, students should be asked to calculate the average atomic mass of a given element, given the table of atomic masses. This can be done by adding up the masses of all of the isotopes of the element, and then dividing by the number of isotopes. This will give the student an overall numerical value for the average atomic mass of the element.
To further explore the concept of average atomic mass, students can be asked to compare the average atomic mass of two elements, given the table of atomic masses. This can be done by calculating the difference between the two average atomic masses, and then determining which element has the higher average atomic mass. Students should also be asked to explain why one element has a higher average atomic mass than the other.
Finally, students can be asked to explain why the average atomic mass of an element is useful. This question will help them to understand why it is important to study the average atomic mass of an element, and how it can be used in research and industry.
By completing these activities on a worksheet, students can gain a better understanding of the concept of average atomic mass, and how it is used in chemistry. Additionally, they will develop critical thinking skills and be able to explain the importance and usefulness of this concept.
Incorporating an Average Atomic Mass Worksheet into Your Chemistry Curriculum for Maximum Effect
Incorporating an Average Atomic Mass Worksheet into a chemistry curriculum can be a powerful tool for student learning. By providing students with an organized and visual approach to average atomic mass calculations, it can help them develop a better understanding of the concept and its real-world applications.
For maximum effectiveness, the worksheet should be implemented at the appropriate point in the curriculum. It should be used to reinforce concepts that students have already learned, such as the periodic table, atomic mass, and isotopes. This will ensure that students have the necessary knowledge to properly complete the worksheet and appreciate its value.
In addition, it is important to provide clear instructions and expectations for students. Explain the purpose of the worksheet and how it can help them understand average atomic mass. Provide guidelines for how to fill out the worksheet and any other helpful tips. Also, explain the grading criteria and how students will be assessed on their work.
Finally, it is essential to incorporate the worksheet into the classroom in an engaging and interactive manner. Give students the opportunity to work together in pairs or small groups to complete the worksheet. This will help them to collaborate and problem-solve together. In addition, provide ample time for students to ask questions and get help if needed.
By carefully planning and executing the use of an average atomic mass worksheet in a chemistry curriculum, it can become an invaluable resource for student learning. Through its implementation, students will have the opportunity to gain a deeper understanding of the concept and its applications and become more confident in their abilities.
Conclusion
The Average Atomic Mass worksheet is a great tool for learning about the properties of atoms and their average atomic masses. It can help students understand the concept of atomic mass, the various elements and their average atomic masses, and the concept of isotopes. By completing the worksheet, students can gain a better understanding of the basic structure and properties of atoms.
[addtoany]