Exploring Atoms Through Hands-On Worksheets: A Middle School Science Curriculum
Teaching Atoms and Molecules: A Middle School Science Worksheet
Atoms and molecules are the building blocks of all matter. This middle school science worksheet provides students with an introduction to the concept of atoms and molecules. It explains the properties of each, as well as the differences between them.
This worksheet begins by introducing the concept of an atom. It explains that an atom is the smallest particle of a chemical element that can exist in nature. Atoms are composed of protons, neutrons, and electrons. It also explains that atoms are too small to be seen by the naked eye, but they are the building blocks of all matter.
The worksheet then introduces the concept of a molecule. It explains that a molecule is a group of two or more atoms held together by chemical bonds. It also explains that molecules can vary in size and shape. A molecule can be made up of two or more of the same type of atom, such as oxygen or hydrogen, or it can be made up of different types of atoms, such as carbon dioxide.
[toc]
The worksheet then compares and contrasts atoms and molecules. It explains that atoms are the smallest particle of a chemical element, while molecules are groups of two or more atoms held together by chemical bonds. It also explains that atoms are not visible to the naked eye, but molecules are visible under a microscope.
The worksheet concludes by providing a few examples of atoms and molecules. It explains that oxygen is an atom, while water is a molecule. It also explains that nitrogen is an atom, while nitric acid is a molecule.
This worksheet is a great introduction to the topic of atoms and molecules for middle school students. It provides an overview of the properties and differences between atoms and molecules, as well as some examples of each. With this worksheet, students will gain a better understanding of the building blocks of all matter.
Structures of Atoms: An Interactive Middle School Science Worksheet
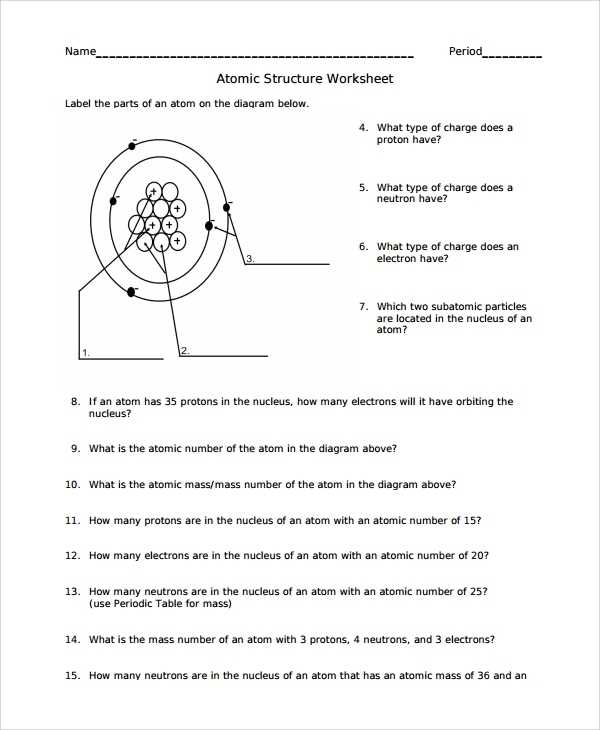
This interactive middle school science worksheet is designed to help students gain a better understanding of the structure of atoms. It will help students identify key atomic components and how they interact with each other.
Atoms are the building blocks of our universe and are composed of three primary components: protons, neutrons, and electrons. Protons are positively charged particles that reside in the nucleus of an atom. Neutrons are neutral particles that also reside in the nucleus. Electrons are negatively charged particles that orbit the nucleus.
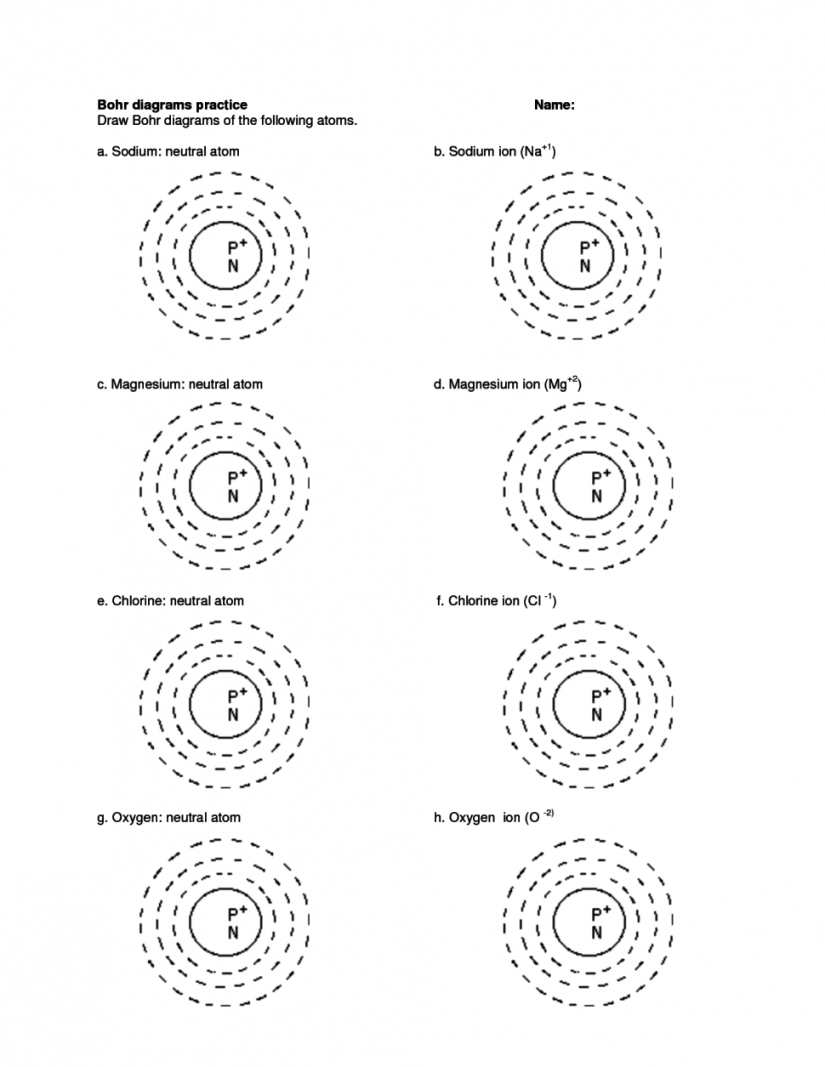
This worksheet allows students to explore the structure of atoms in an interactive way. It starts by introducing the different components of an atom and their respective charges. Then, students are asked to draw a diagram of an atom, labeling the protons, neutrons, and electrons.
Next, students are asked to identify the number of protons, neutrons, and electrons in a given atom. They will also be asked to calculate the number of neutrons in a given atom given the number of protons and electrons.
Finally, students are asked to explain how the different components of an atom interact with each other. This section of the worksheet encourages students to think critically about the structure of atoms and its implications for the chemical properties of different elements.
By the end of this worksheet, students will have a better understanding of atomic structure and the role of its components in determining the properties of elements. They will have a deeper appreciation for the fundamental building blocks of our universe.
Understanding Atoms: A Comprehensive Middle School Science Worksheet
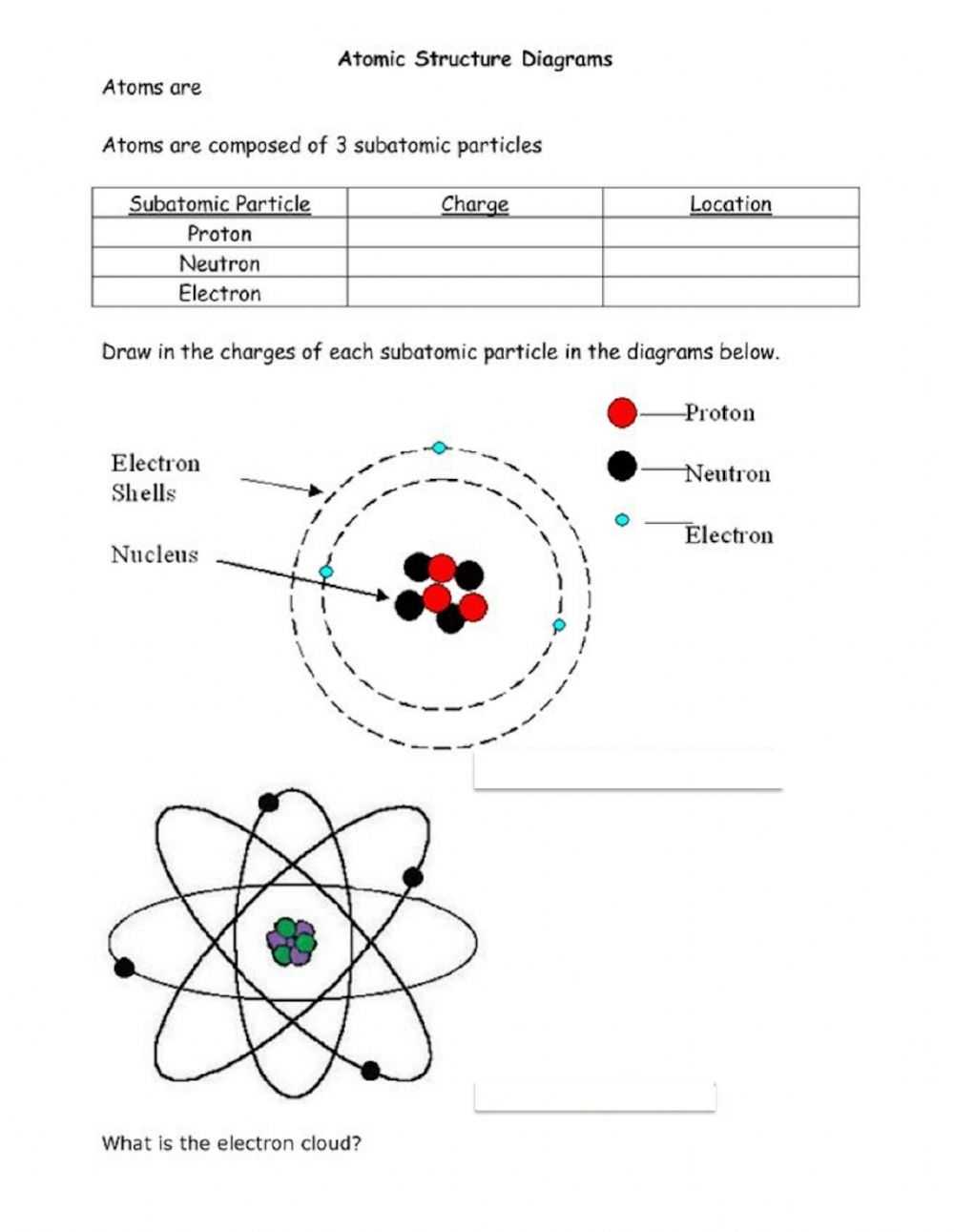
Atoms are the building blocks of matter and are the smallest particles that make up all material substances. Understanding atoms and their structure is a fundamental concept in middle school science. This worksheet will help students comprehend and interpret the structure of atoms.
First, it is important to note that atoms are made up of three major parts: protons, neutrons, and electrons. Protons and neutrons are located in the nucleus of the atom, while electrons orbit around the nucleus in shells. In order to understand atoms, it is important to understand the properties of each of these parts.
Protons are positively charged particles. The number of protons in an atom determines what type of element it is; for example, all hydrogen atoms have one proton, all oxygen atoms have 8 protons, and all gold atoms have 79 protons. The number of protons in an atom also determines its atomic number, which is the number that appears on the periodic table.
Neutrons are particles with no charge and help to hold the nucleus together. The number of neutrons in an atom can vary, but the number of neutrons for a specific element is usually the same. For example, oxygen atoms can have 8, 9, or 10 neutrons, but the most common type of oxygen atom has 8 neutrons.
Electrons are negatively charged particles that orbit around the nucleus in shells. The number of electrons determines how much electricity an atom can carry, so it is important to understand this relationship. Additionally, electrons determine how an atom will interact with other atoms.
In conclusion, understanding the structure of atoms is an important part of middle school science. This worksheet has provided a basic overview of the three major parts of atoms, their properties, and how they interact with each other. By understanding this information, students will be better prepared to comprehend more complex concepts related to atoms and their structure.
Conclusion
In conclusion, atoms are the basic building blocks of all matter. Through this worksheet, middle school students were able to learn some of the basics of atoms, including their structure and the different kinds of particles that make them up. This knowledge can help them to better understand the world around them and how it works on a microscopic level.
[addtoany]