Exploring the Various Components of an Atomic Structure Worksheet Answers Key
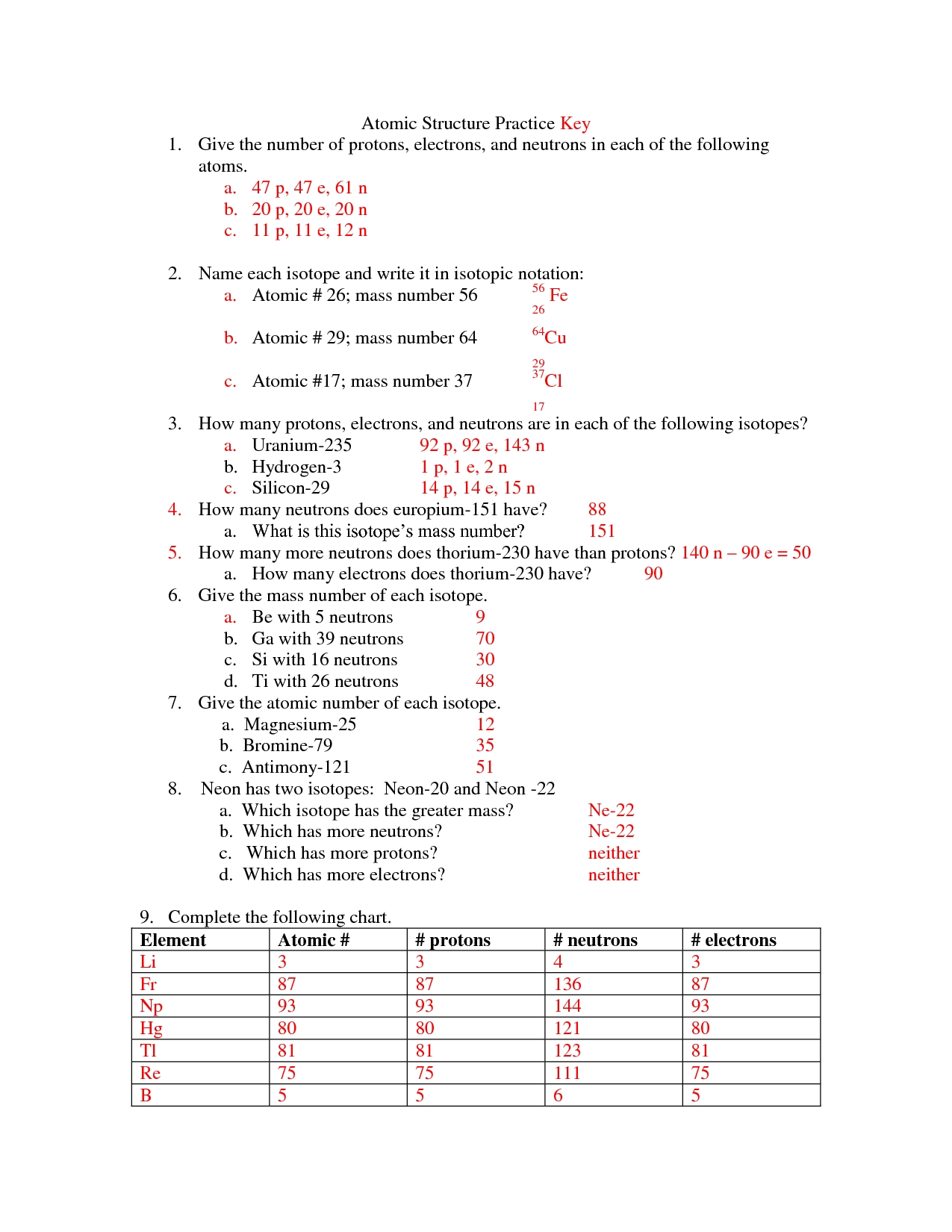
1. What is an atom?
An atom is a basic unit of matter that is composed of a nucleus containing protons and neutrons, surrounded by electrons in a cloud-like orbital pattern. Atoms are the building blocks of all matter, and the properties of an element are determined by the number of protons in the nucleus.
2. What are the components of an atom?
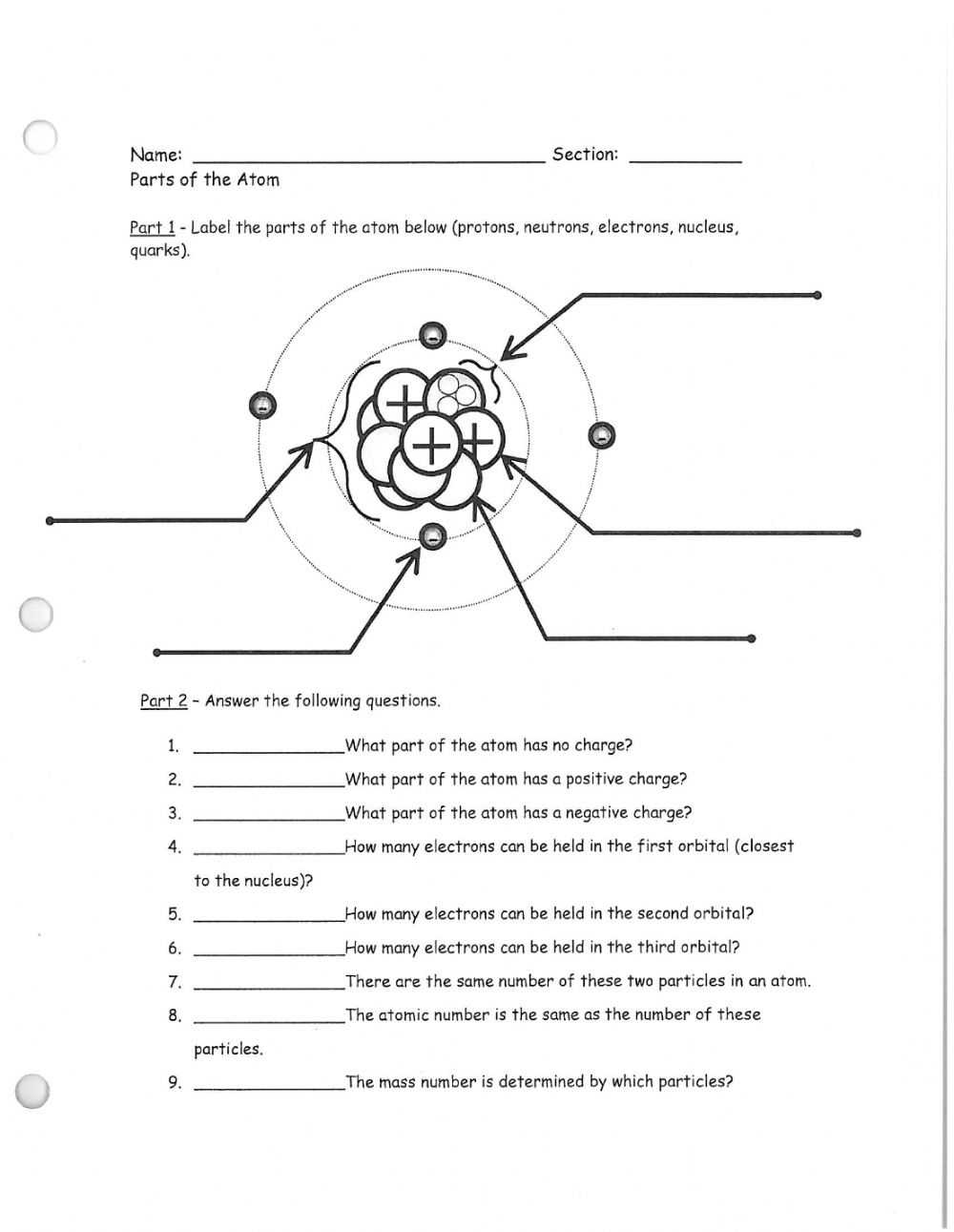
The components of an atom include a nucleus, protons, neutrons, and electrons. The nucleus is the center of the atom, and it contains protons and neutrons. Protons carry a positive charge and neutrons carry no charge. Electrons are located outside of the nucleus, and they carry a negative charge.
3. What is the role of the nucleus?
The nucleus is the center of an atom, and it is composed of protons and neutrons. The nucleus is responsible for the properties of an element, as it determines the number of protons in the atom. The number of protons determines the chemical properties of the element.
[toc]
4. What is the role of protons?
Protons are particles that are located in the nucleus of an atom, and they carry a positive charge. Protons are responsible for determining the properties of an element, as the number of protons determines the chemical properties of the element.
5. What is the role of neutrons?
Neutrons are particles that are located in the nucleus of an atom, and they carry no charge. Neutrons are responsible for helping keep the nucleus stable, as they help to balance out the positive charge of the protons.
6. What is the role of electrons?
Electrons are particles that are located outside of the nucleus of an atom, and they carry a negative charge. Electrons are responsible for forming chemical bonds between atoms, as they can be shared or transferred between atoms. Electrons are also responsible for the electrical properties of an atom.
A Comprehensive Guide to Understanding Nuclear Chemistry Through Atomic Structure Worksheet Answers Key
Introduction
Nuclear chemistry is a complex and fascinating field of study. It involves the study of the structure and behavior of atoms and the interactions between them. Nuclear chemistry is closely related to atomic structure, and understanding the structure of atoms is essential for understanding nuclear chemistry. This guide will provide an overview of atomic structure, including the components of an atom and how they interact, and explain how this knowledge can be applied to understanding nuclear chemistry.
Atomic Structure
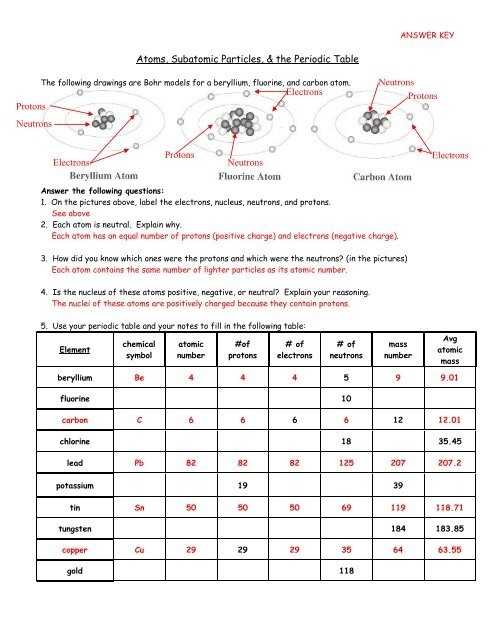
Atoms are composed of three main components: protons, neutrons, and electrons. Protons and neutrons make up the nucleus of an atom, while electrons orbit around the nucleus. Protons have a positive charge, while neutrons have no charge. Electrons have a negative charge.
The number of protons in an atom is referred to as its atomic number. This determines the type of element that the atom is. The number of neutrons in an atom is referred to as its mass number. This determines the isotope of the element. Different isotopes of an element can have different properties.
Interactions Between Atoms
Atoms can interact with each other in a variety of ways. The most common types of interactions are chemical bonds, which involve the sharing of electrons between atoms. There are also electromagnetic forces, which are responsible for the attraction and repulsion between atoms.
Nuclear Chemistry
Nuclear chemistry is the study of the structure and behavior of atoms and the interactions between them. It is closely related to atomic structure, and understanding the structure of atoms is essential for understanding nuclear chemistry. Nuclear chemistry involves the study of radioactive decay, nuclear reactions, nuclear energy, and more.
Conclusion
Atomic structure plays a crucial role in understanding nuclear chemistry. This guide has provided an overview of atomic structure, including the components of an atom, how they interact, and how this knowledge can be applied to understanding nuclear chemistry. With a better understanding of atomic structure, it is possible to gain a deeper understanding of nuclear chemistry.
Analyzing Atomic Structure Through Practice Questions and Answers: An Atomic Structure Worksheet Answers Key
I. Multiple Choice Questions
A. What is the number of protons in a carbon atom?
Answer: 6
B. What is the number of neutrons in a carbon atom?
Answer: 6
C. What is the number of electrons in a carbon atom?
Answer: 6
D. What is the atomic number of an atom with 6 protons?
Answer: 6
E. What is the mass number of an atom with 6 protons and 6 neutrons?
Answer: 12
F. What is the atomic mass of an atom with 6 protons and 6 neutrons?
Answer: 12 amu (atomic mass units)
II. True or False
A. The number of protons in an atom determines its atomic number.
Answer: True
B. The number of neutrons in an atom determines its mass number.
Answer: True
C. The number of electrons in an atom determines its atomic mass.
Answer: False (the number of protons and neutrons determines its atomic mass)
Unpacking the Fundamental Concepts of Atomic Structure Through Atomic Structure Worksheet Answers Key
Atomic structure is a fundamental concept of chemistry that has far-reaching implications for the study of matter. By understanding the structure of an atom, we can better comprehend the behavior of molecules, chemical reactions, and other aspects of the physical sciences. To help students gain a better understanding of atomic structure, an atomic structure worksheet is a valuable tool.
The atomic structure worksheet answers key provides an organized and comprehensive overview of the concepts associated with atomic structure. It begins with a definition of an atom, highlighting its positive and negative charges and its nucleus. It then moves on to discuss the different types of subatomic particles, such as protons, neutrons, and electrons, and the roles they play in an atom. It also explains the atomic number, isotopes, and atomic orbitals.
Next, the worksheet answers key examines the structure of the nucleus and its components, including the protons, neutrons, and electrons. It also explains the process of nuclear fusion, radioactive decay, and the formation of different isotopes. Additionally, the answers key explains the different types of bonds, such as ionic, covalent, and metallic, and the forces that hold them together.
Finally, the atomic structure worksheet answers key covers the properties of atoms and the periodic table. It explains the different types of elements, their atomic numbers, and their properties. It also explains the different types of compounds and the role of electrons in chemical reactions.
By unpacking the fundamental concepts of atomic structure through the atomic structure worksheet answers key, students can gain a comprehensive understanding of the subject. The organized format and comprehensive coverage makes the worksheet a valuable resource for any student seeking to gain a better understanding of atomic structure.
Conclusion
The Atomic Structure Worksheet Answers Key provides a valuable resource for students to understand the fundamentals of atomic structure. Through this resource, students can learn the basics of atomic structure, how atoms interact with each other, and how to use the information to solve problems. With this knowledge, students can gain a deeper understanding of chemistry and its related topics.
[addtoany]