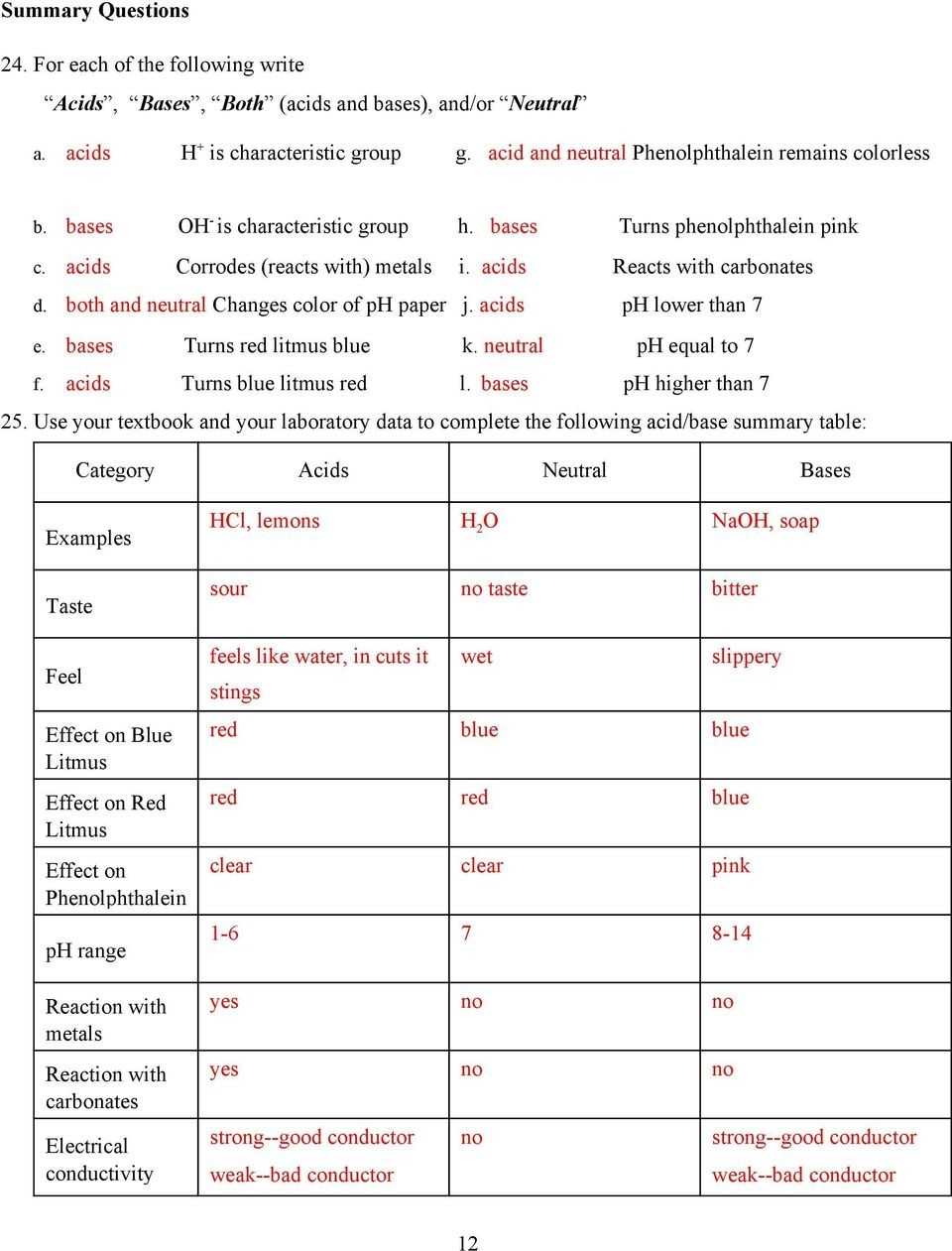
Unpacking Acids and Bases Worksheet Answers: A Comprehensive Guide
Acids and bases are important components of chemical reactions and understanding their properties is essential for any student of chemistry. Acids and bases can be identified by a variety of characteristics, including taste, reactivity, and concentration. This worksheet will help students understand the different properties of acids and bases and how they can be identified.
1. What is an acid?
An acid is a substance that releases hydrogen ions (H+) when dissolved in water. It has a sour taste, and can turn litmus paper red. Acids are corrosive and reactive and can be found in a variety of products, such as vinegar, lemon juice, and battery acid.
[toc]
2. What is a base?
A base is a substance that accepts hydrogen ions (H+) when dissolved in water. It has a bitter taste, and can turn litmus paper blue. Bases are slippery to the touch and can be found in a variety of products, such as baking soda, soap, and antacids.
3. What is the pH scale and what does it measure?
The pH scale is a measure of the acidity or alkalinity of a solution. It ranges from 0 to 14, with 7 being neutral. Lower pH values indicate increasing acidity, while higher pH values indicate increasing alkalinity.
4. What are indicators and what are they used for?
Indicators are substances that change color in response to changes in pH. They are used to measure the acidity or alkalinity of a solution. Common indicators include litmus paper, phenolphthalein, and bromothymol blue.
5. What is the difference between a strong acid and a weak acid?
A strong acid is one that is highly concentrated and quickly releases hydrogen ions when dissolved in water. A weak acid is one that is less concentrated and releases hydrogen ions more slowly when dissolved in water.
Exploring the Properties of Acids and Bases with Worksheet Answers
Acids and bases are important chemical compounds that can be found in everyday life. Acids have a sour taste and react with metals to produce hydrogen gas. Bases are usually slippery or soapy to the touch and react with acids to produce a neutral solution. In order to understand the properties of acids and bases, it is essential to use worksheet answers to help guide the learning process.
Worksheet answers can be used to help explore the properties of acids and bases. For example, one worksheet can ask the student to identify the pH of a solution of an acid and a base. The student can then answer the question by analyzing the properties of the two compounds. By understanding how they interact with each other, the student can determine the pH of the solution.
Another worksheet can ask the student to identify the strength of an acid or base. In order to answer this question, the student must understand the concept of acidity and basicity. By looking at the concentration of the acid or base, they can determine how strong it is.
Worksheet answers can also be used to explore the effects of mixing different acids and bases. For example, the student can identify the end product of a reaction between two different compounds. By understanding the chemical processes involved in the reaction, the student can gain an understanding of how the reaction will affect the final product.
In addition to exploring the properties of acids and bases, worksheet answers can also help students understand the role of indicators in determining pH. By understanding how indicators work, the student can learn how to test for pH levels. Indicators are substances that change color when exposed to an acid or base. By understanding how an indicator works, the student can understand how pH is determined.
Worksheet answers can also be used to help students explore the reaction between an acid and a base. The student can identify the products of the reaction and analyze how the reaction affects the final product. By understanding the chemical processes involved in the reaction, the student can gain an understanding of how the reaction will affect the final product.
Worksheet answers are a great way to help students explore the properties of acids and bases. By understanding how the acids and bases interact with each other, students can gain an understanding of how they can use them in everyday life. Understanding the properties of acids and bases can help students better understand the science behind everyday activities.
Understanding Acids and Bases Worksheet Answers: A Step-by-Step Guide
Acids and bases are essential components in many chemical reactions. Understanding the nature of these compounds is essential for anyone studying chemistry. This worksheet provides a step-by-step guide to help you understand the fundamentals of acids and bases.
1. What are acids and bases?
Acids are compounds that have a sour taste and a pH below 7. They are generally proton donors, meaning they release hydrogen ions (H+) when dissolved in water. Bases are compounds that have a bitter taste and a pH above 7. They are generally proton acceptors, meaning they accept hydrogen ions (H+) when dissolved in water.
2. What is the difference between a strong acid and a weak acid?
Strong acids are compounds that completely dissociate or ionize in water, releasing a large number of hydrogen ions (H+). Weak acids only partially dissociate or ionize in water, releasing fewer hydrogen ions (H+).
3. What is the difference between a strong base and a weak base?
Strong bases are compounds that completely dissociate or ionize in water, accepting a large number of hydrogen ions (H+). Weak bases only partially dissociate or ionize in water, accepting fewer hydrogen ions (H+).
4. What is the difference between an acid base reaction and a neutralization reaction?
An acid base reaction occurs when an acid and base react with each other to form a new compound. This reaction often produces heat and is often accompanied by a color change. A neutralization reaction is an acid base reaction in which an acid and a base react with each other to form a salt and water. This reaction is often accompanied by a color change and the release of heat.
5. What is the pH scale?
The pH scale is used to measure the acidity or alkalinity of a solution. The scale ranges from 0 (very acidic) to 14 (very basic). A pH of 7 is considered neutral. Solutions with a pH below 7 are considered acidic and solutions with a pH above 7 are considered basic.
Conclusion
The Acids and Bases Worksheet Answers provide a great starting point for understanding acids and bases and the role they play in everyday life. It is important to remember that acids and bases have different characteristics, and that there are many different types of each. By understanding the different characteristics of acids and bases, it is possible to make educated decisions about the use of these substances in everyday life.
[addtoany]