Exploring the Benefits of Understanding Polarity of Bonds in Chemistry: A Worksheet Answer Guide
Understanding the polarity of bonds in chemistry is an essential part of the study of chemical reactions and properties. This knowledge can provide valuable insights into how different compounds interact and react with one another, enabling students to better understand the principles of chemistry. In this worksheet answer guide, we will explore the benefits of understanding the polarity of bonds in chemistry and discuss how this knowledge can be applied to real-world scenarios.
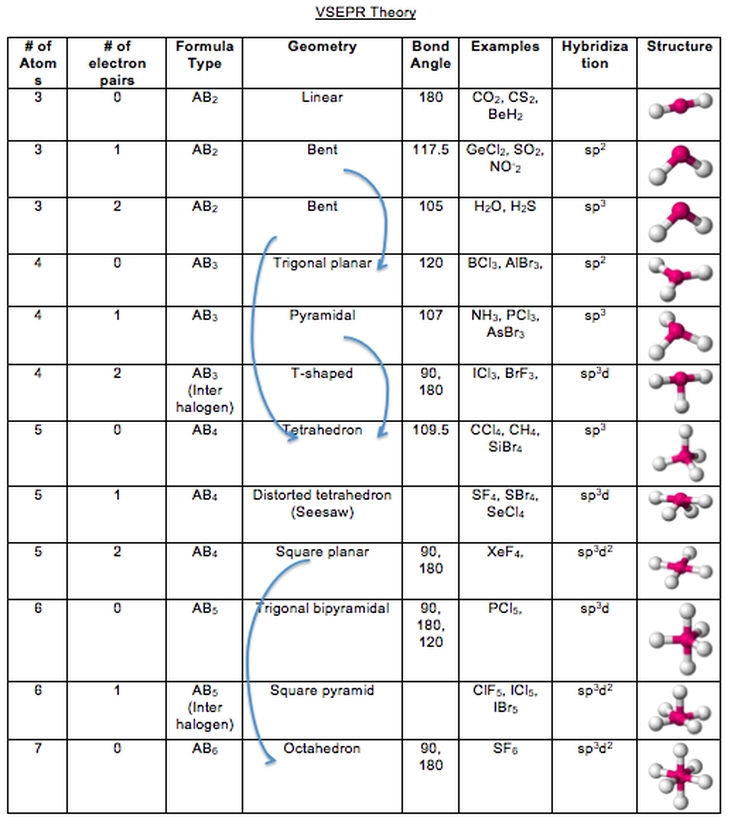
In chemistry, a bond is formed when two atoms share a pair of electrons. The polarity of a bond is determined by the difference in electronegativity between the atoms that form the bond. If the atoms have similar electronegativities, the bond is considered nonpolar. If the atoms have different electronegativities, the bond is considered polar. Polar bonds will have a positive and negative end, while nonpolar bonds will be neutral.
One of the primary benefits of understanding the polarity of bonds in chemistry is the ability to predict how different compounds will interact with one another. Polar bonds are more likely to attract one another, while nonpolar bonds are more likely to repel one another. By knowing the polarity of the bonds in a compound, chemists can predict how different compounds will react with one another. This knowledge can be applied to many real-world scenarios, such as when designing new medications or analyzing the interactions between different chemicals.
[toc]
Additionally, understanding the polarity of bonds can be useful for analyzing the structure of a compound. Polar bonds tend to align in a particular direction, which can be used to determine the three-dimensional shape of a molecule. This information can be used to predict how the molecule will interact with other molecules, as well as how it will react to different environmental conditions.
Finally, understanding the polarity of bonds can be useful for understanding the properties of a compound. Polar compounds tend to be more soluble in water, while nonpolar compounds tend to be less soluble. Knowing the polarity of a compound can provide an indication of its solubility and other physical properties, such as boiling point and melting point.
In summary, understanding the polarity of bonds in chemistry can provide valuable insights into how different compounds interact and react with one another. This knowledge can be applied to many real-world scenarios, such as designing new medications and analyzing the structure and properties of compounds. With this worksheet answer guide, we have explored the benefits of understanding the polarity of bonds in chemistry, and discussed how this knowledge can be applied to a variety of scenarios.
Strategies for Teaching Polarity of Bonds for Greater Comprehension: A Worksheet Answer Guide
1. Start by introducing the concept of polarity.
Polarity is an important concept in chemistry that describes the unequal sharing of electrons between atoms in a molecule. This unequal sharing of electrons creates a partial positive and partial negative charge that results in a molecule having a specific overall charge. The charge will depend on how the electrons are distributed, and understanding this concept is key to understanding how various molecules interact with each other.
2. Explain what a polar bond is.
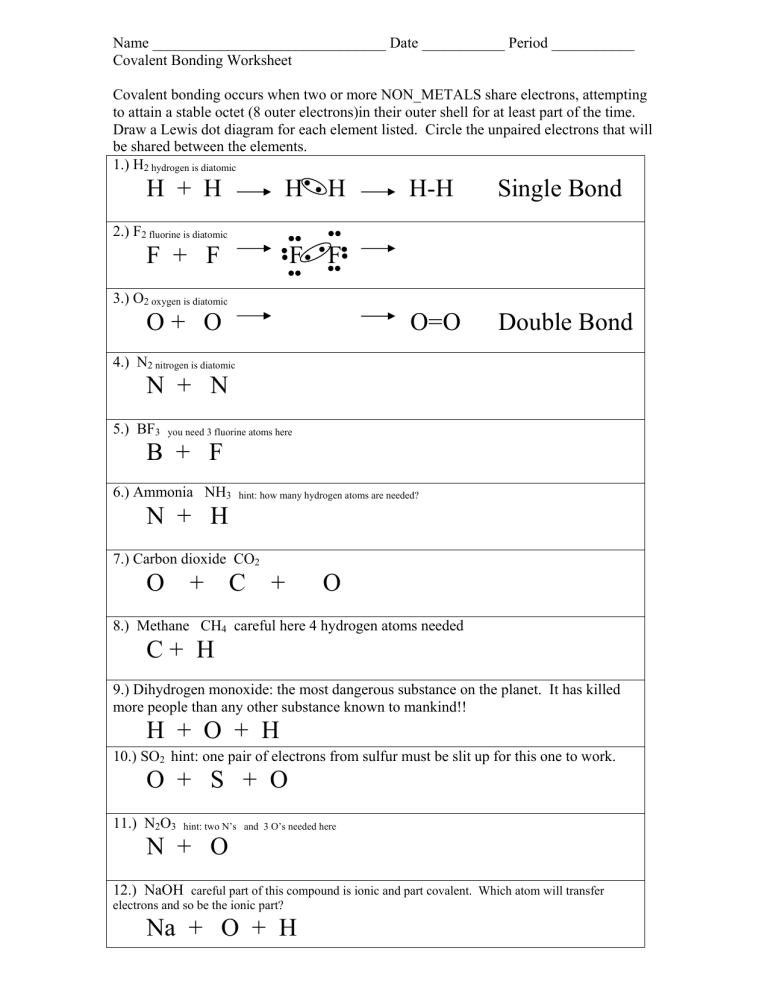
A polar bond is a type of bond in which the electrons are not shared equally between atoms. This creates an imbalance of charge, with one end of the molecule having a partial positive charge and the other having a partial negative charge. This imbalance of charge causes the molecule to be attracted to other molecules that have the opposite charge. By understanding the concept of polar bonds, it is easier to understand how various molecules interact with each other and how they can be used in different applications.
3. Provide examples of polar bonds.
Examples of polar bonds include water molecules, which contain two hydrogen atoms and one oxygen atom. The oxygen atom has a higher electronegativity than the hydrogen atoms, so it pulls the electrons more towards itself. This creates a partial negative charge on the oxygen atom and a partial positive charge on the hydrogen atoms, making the water molecule polar. Another example of a polar bond is the bond between a hydrogen atom and an oxygen atom in a hydrogen peroxide molecule. Again, the oxygen atom has a higher electronegativity, creating a partial negative charge on the oxygen atom and a partial positive charge on the hydrogen atom.
4. Explain why it is important to understand polarity of bonds.
Understanding the concept of polarity is important because it helps us understand how various molecules interact with each other. By knowing how the electrons are distributed in a molecule, we can predict how it will interact with other molecules, which is important for many applications, including drug design, materials science, and biochemistry. Knowing the polarity of a molecule also helps us understand why certain molecules are soluble in certain solvents and why others are not. Understanding polarity also helps us to design molecules with specific properties, such as increased solubility or increased reactivity.
How to Use Polarity of Bonds to Solve Challenging Chemistry Problems: A Worksheet Answer Guide
Using polarity of bonds to solve challenging chemistry problems can be a difficult task. However, with the right approach, it can become a much easier task. This worksheet answer guide provides a step-by-step approach to solving challenging chemistry problems using the concept of polarity of bonds.
Step 1: Understand the concept of polarity of bonds.
Polarity of bonds is a concept that describes how the electrons in a molecule or ion are shared or distributed. When the electrons are shared or distributed unevenly, the bond is said to be polar. Polar bonds have a positive end and a negative end. This means that the molecule or ion has an overall positive and negative charge.
Step 2: Identify the charges of atoms in the molecule or ion.
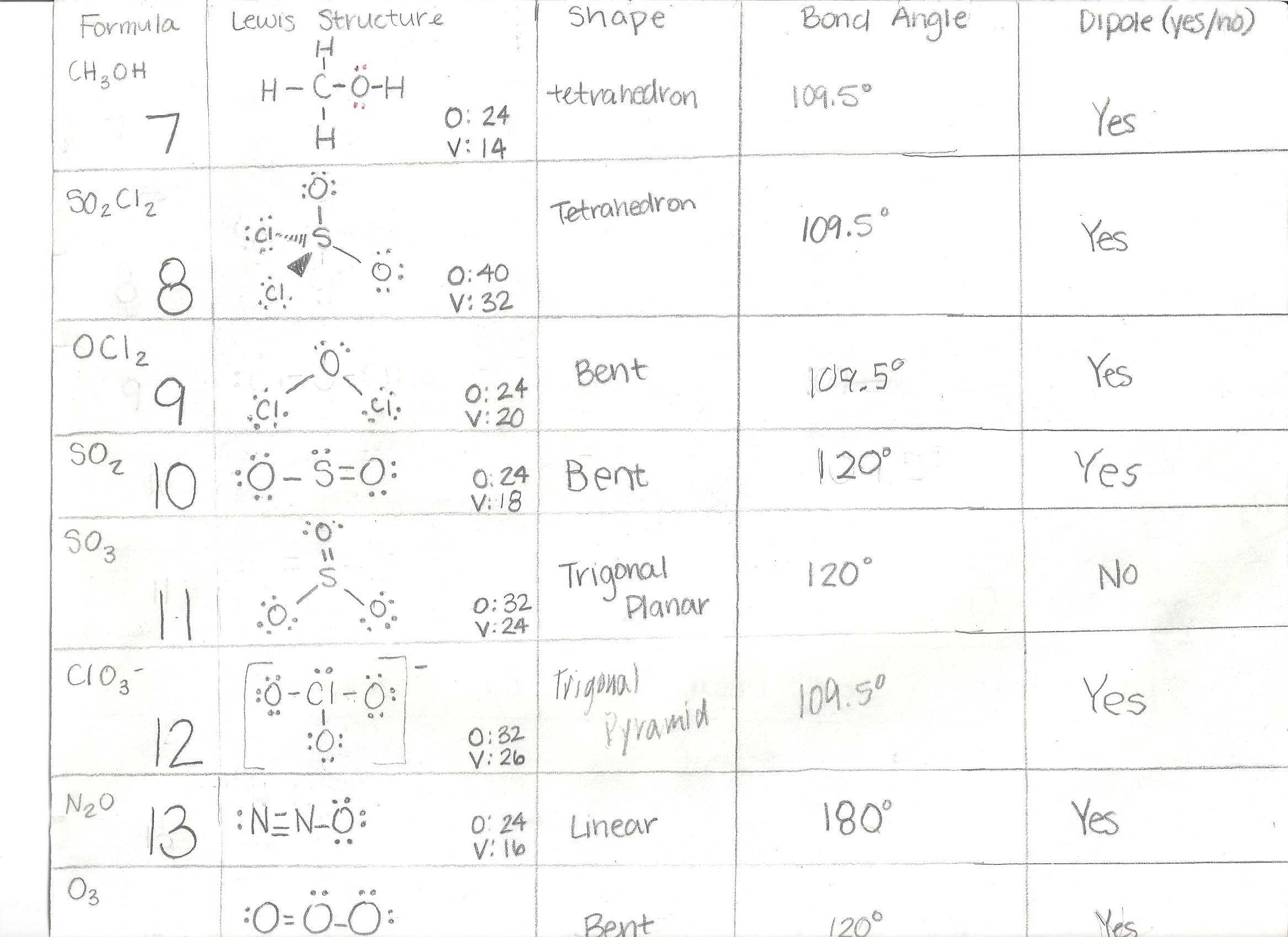
The first step in solving a challenging chemistry problem using the concept of polarity of bonds is to identify the charges of atoms in the molecule or ion. This can be done by looking at the Lewis structure of the molecule or ion and determining the number of electrons shared or distributed between each atom. The number of electrons shared or distributed will determine the charge of each atom.
Step 3: Determine the polarity of the bond.
Once the charges of each atom have been identified, the polarity of the bond can be determined. If the charges of the atoms are different, then the bond is polar. If the charges of the atoms are the same, then the bond is non-polar.
Step 4: Use the polarity of the bonds to solve the problem.
Once the polarity of the bonds has been determined, the polarity of the bonds can be used to solve the problem. The polarity of the bonds can be used to determine the overall charge of the molecule or ion, which can then be used to determine what type of reaction will occur.
By following these steps, challenging chemistry problems can be solved using the concept of polarity of bonds. With this approach, understanding and utilizing polarity of bonds can become a much easier task.
Conclusion
The answers to the worksheet on the polarity of bonds demonstrate the importance of understanding the polarity of bonds in chemistry. This understanding is essential for accurately predicting the physical and chemical properties of molecules. Knowing how to interpret the polarity of bonds can help chemists to better understand the behavior of molecules and can provide insight into the chemical processes taking place.
[addtoany]