Exploring Different States of Matter with a States of Matter Worksheet Chemistry Activity
Exploring the different states of matter is a fascinating endeavor that requires a thorough understanding of the physical and chemical properties of matter. This activity utilizes a states of matter worksheet to explore the different states of matter and their properties.
The worksheet presents a series of questions that can be used to examine the different characteristics of the states of matter. The questions address the differences in density, boiling point, and melting point between the various states of matter. Additionally, the worksheet explores the changes in state that can occur when a substance is heated or cooled.
By answering the questions on the worksheet, students gain a better understanding of the physical and chemical properties of the different states of matter. They also gain a better understanding of the physical and chemical processes that can cause a change in state.
[toc]
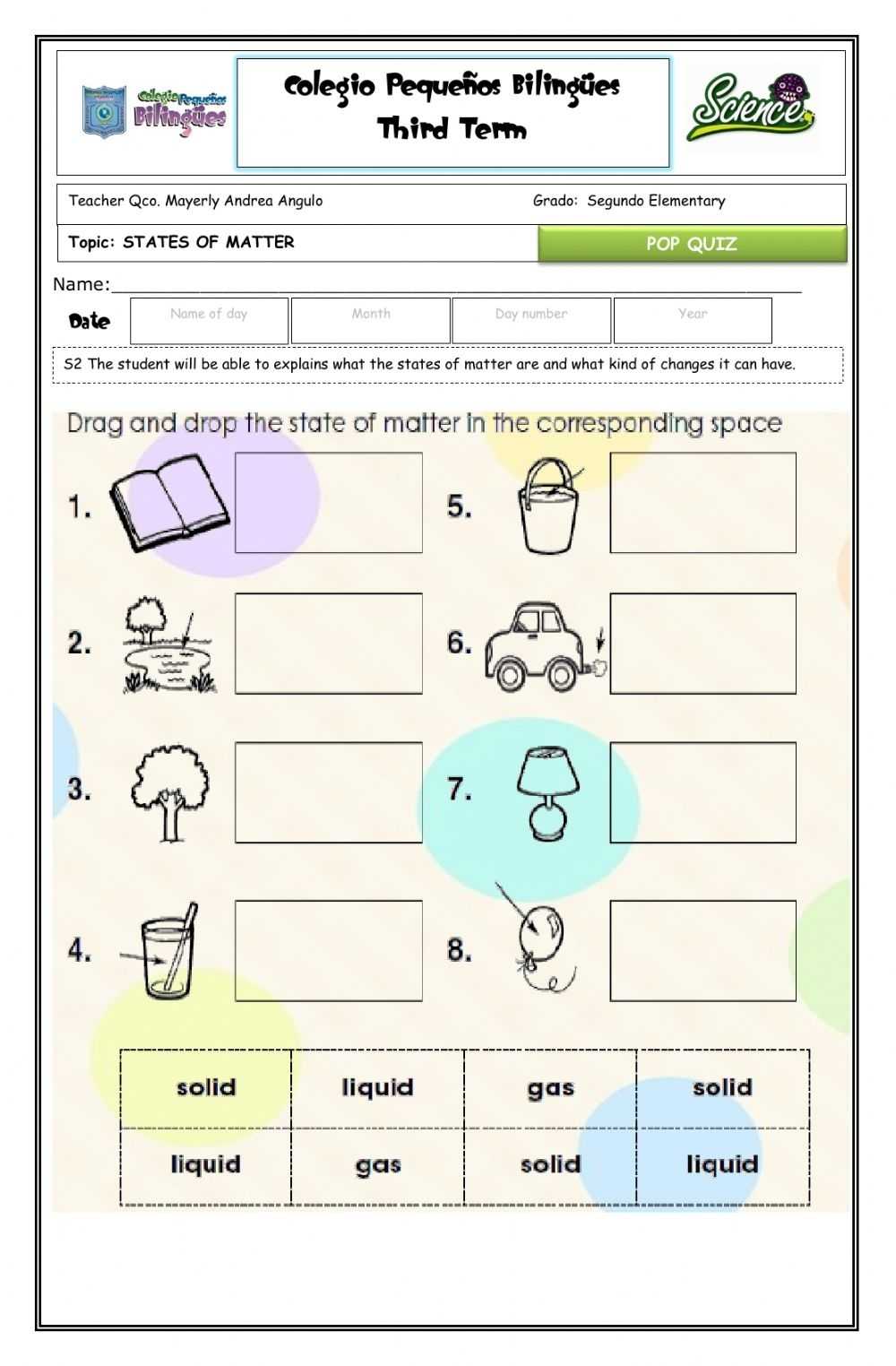
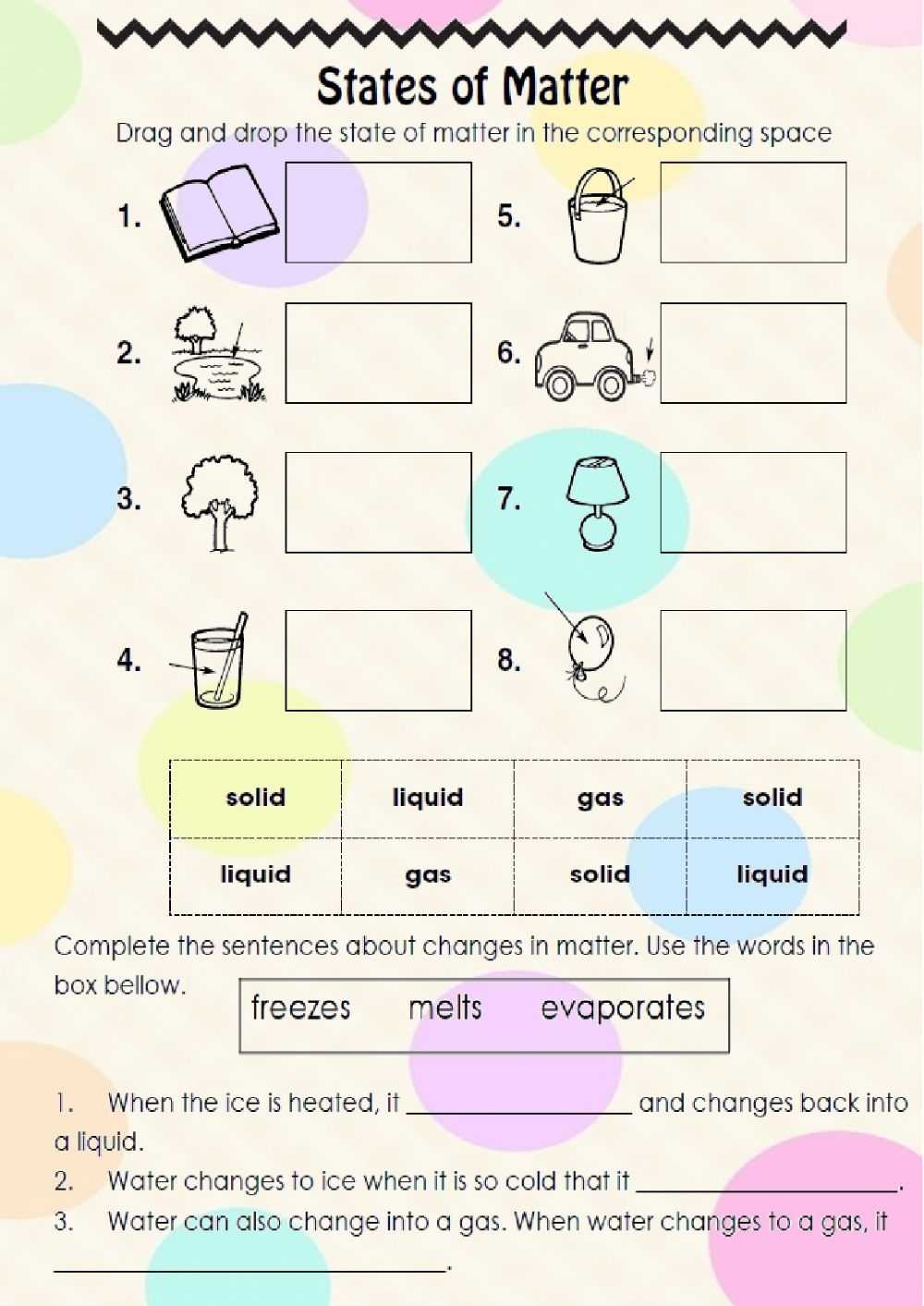
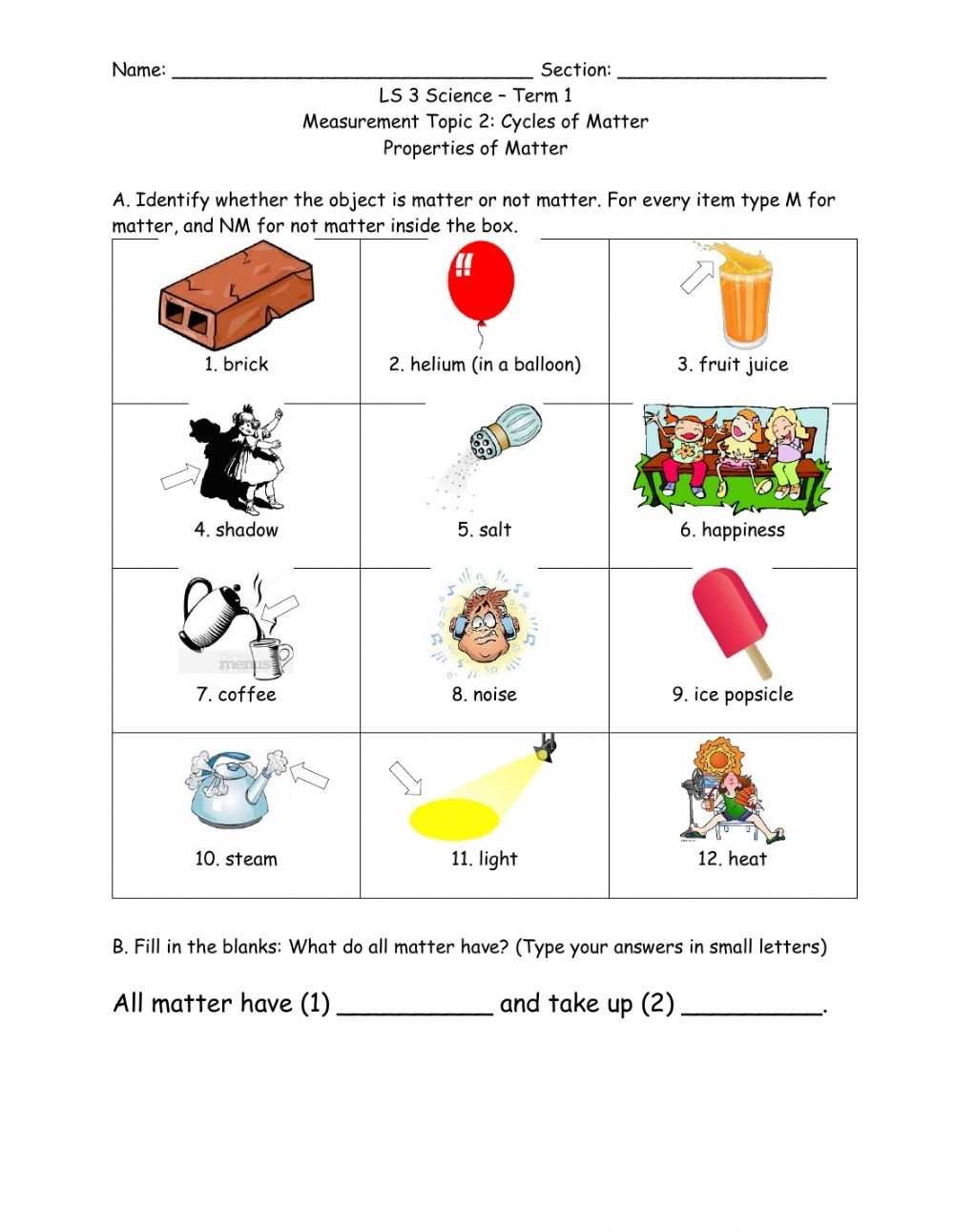
The worksheet also provides diagrams and diagrams of the different states of matter. This helps students to visualize the differences between the states and gain an understanding of the properties that determine their states.
By completing this worksheet, students gain a better understanding of the different states of matter and the processes that lead to changes in state. They also gain a better understanding of the physical and chemical properties of the various states of matter.
Investigating Intermolecular Forces with States Of Matter Worksheet Chemistry Exercises
Investigating intermolecular forces is an essential part of chemistry. Intermolecular forces are the forces of attraction that exist between molecules, and they are important to understand in order to better comprehend how molecules interact with each other. In this exercise, we will explore how intermolecular forces affect the states of matter.
In order to understand how intermolecular forces affect the states of matter, we must first understand the types of intermolecular forces. These forces can be divided into two main categories: ionic and van der Waals forces. Ionic forces are those that exist between ions and are related to electrostatic attraction. Van der Waals forces, on the other hand, are caused by the attraction between molecules due to their dipole moments.
The next step is to examine how these forces affect the states of matter. Generally, the stronger the intermolecular forces, the higher the melting and boiling points of a substance. Therefore, a substance with strong intermolecular forces will be more likely to remain in a solid or liquid state, while one with weaker intermolecular forces will be more likely to exist in a gaseous state.
This can be further illustrated by looking at examples from various substances. For instance, water has strong intermolecular forces, so it remains in a liquid state at room temperature. By contrast, hydrogen and helium, which both have weak intermolecular forces, exist in a gaseous state at room temperature.
Finally, it is important to note that the intermolecular forces also affect the properties of a substance. For example, substances with strong intermolecular forces tend to be more viscous, as the forces make it harder for the molecules to move past each other. In addition, substances with strong intermolecular forces tend to have higher boiling points, as it requires more energy to break the attractions between the molecules.
In conclusion, intermolecular forces play an important role in determining the states of matter and properties of a substance. By understanding the types of forces that exist between molecules, we can better comprehend how these forces affect the states of matter, as well as the physical and chemical properties of a substance.
Analyzing the Physical Properties of the Different States of Matter Using States Of Matter Worksheet Chemistry Problems
Matter is a fundamental component of the physical universe and exists in various forms. The different states of matter are solid, liquid, and gas. With the help of a states of matter worksheet chemistry problems, it is possible to analyze the physical properties of each of these states of matter.
The solid state is characterized by particles that are closely packed together and held in position by strong intermolecular forces. As a result, solids have a fixed shape and volume. In general, solids are rigid, incompressible, and insoluble in most liquids. The particles in a solid move very slowly, providing little opportunity for thermal expansion.
The liquid state is characterized by particles held together by much weaker intermolecular forces. As a result, liquids have a fixed volume but no fixed shape and can take the shape of any container. Liquids are also compressible, soluble in most other liquids, and capable of thermal expansion. The particles in a liquid move more quickly than those in a solid, giving liquids a greater capacity for thermal expansion.
The gas state is characterized by particles held together by negligible intermolecular forces. As a result, gases have no fixed shape or volume and can expand or contract to fill any container. Gases are compressible, soluble in most other gases, and highly capable of thermal expansion. The particles in a gas move quickly, allowing for rapid thermal expansion.
By using a states of matter worksheet chemistry problems, it is possible to analyze the physical properties of each of these states of matter. This analysis can provide insight into the behavior of matter and the interactions between particles in each state. In addition, this analysis can also help to explain why certain materials exist in one state of matter and not another.
Conclusion
This States of Matter Worksheet Chemistry has provided students with a great insight into the fundamental concepts of the scientific study of matter. Through the use of diagrams and questions, students have been able to gain a better understanding of the different states of matter, as well as the differences between them. With this knowledge, students are now better equipped to answer questions and complete assignments related to this subject.
[addtoany]