How to Use Simple Binary Ionic Compounds Worksheets to Master Chemical Reactions
Mastering chemical reactions can be a daunting task, but with the use of simple binary ionic compounds worksheets, it can be mastered with relative ease. Binary ionic compounds are composed of two elements, usually a metal and a nonmetal, and are represented by a formula that consists of two elements separated by a plus sign. By working through binary ionic compounds worksheets, students can become familiar with the various elements and their corresponding formulas, making it easier to understand the reactions between them.
To get started, it is important to make sure that the student is familiar with the symbols for each element. These symbols can be found in most periodic tables, and it is essential that the student becomes comfortable with the symbols in order to correctly interpret the formulas. Once the symbols are familiar, students can start to work through the worksheets. Depending on the worksheet, it may ask the student to identify the elements in a given compound, calculate the number of atoms of each element in the compound, or determine the type of reaction between two compounds.
No matter which worksheet is used, it is important to use the information provided to understand the reaction between two elements. After the student has identified the compounds and the reaction between them, they can use the worksheet to calculate the number of ions in the reaction and determine the products of the reaction. This process allows the student to gain a better understanding of the reaction and can help them to remember the reaction for future reference.
[toc]
By utilizing binary ionic compounds worksheets, students can become more proficient in understanding chemical reactions. With practice, the student will soon become comfortable with the symbols and formulas and be able to interpret chemical reactions with relative ease.
Exploring the Different Types of Simple Binary Ionic Compounds and Their Properties
Simple binary ionic compounds are composed of two elements in a chemical formula, usually a metal and a nonmetal. These compounds form when atoms of the two elements exchange electrons, resulting in a transfer of electrical charge. Ionic compounds are held together by the electrostatic attraction between the oppositely charged ions.
The properties of simple binary ionic compounds depend on the type of atoms they are composed of. The metal atoms are typically larger and less electronegative than the nonmetal atoms, making them the cation in the compound. The nonmetal atoms are typically smaller and more electronegative than the metal atoms, making them the anion.
The physical properties of simple binary ionic compounds depend on the type of metal and nonmetal elements they are made of. For example, compounds composed of alkali metals and halogens form white, crystalline solids with high melting points. Compounds composed of transition metals and halogens, on the other hand, form colored, crystalline solids with lower melting points.
The chemical properties of simple binary ionic compounds are also determined by the type of metal and nonmetal elements they are composed of. For instance, compounds composed of alkali metals and halogens tend to be highly reactive and form strong acids when dissolved in water. Compounds composed of transition metals and halogens, however, are less reactive and form weak acids when dissolved in water.
In conclusion, simple binary ionic compounds are composed of two elements that exchange electrons, resulting in a transfer of electrical charge. The properties of these compounds depend on the type of metal and nonmetal atoms they are composed of, with alkali metal-halogen compounds exhibiting different physical and chemical properties than transition metal-halogen compounds.
The Benefits of Understanding Simple Binary Ionic Compounds for Chemistry Students
Understanding simple binary ionic compounds is an important skill for chemistry students to have. These compounds are composed of two elements, a positively-charged cation and a negatively-charged anion, held together by electrostatic forces. By understanding how these compounds are formed and how they interact with each other, students will be better equipped to analyze more complex compounds and compounds found in nature.
First, understanding simple binary ionic compounds helps students understand the fundamentals of chemical bonding. When the cation and anion interact, electrons are transferred from the cation to the anion, forming an ionic bond. This bond is characterized by the attraction between the oppositely charged particles, and its strength is related to the charge of the ions, the radius of the ions, and the distance between them. By understanding how this bond is formed, students will be better equipped to analyze more complex compounds.
Second, understanding simple binary ionic compounds helps students understand how elements interact with each other on a molecular level. By understanding the characteristics of different ions, such as their charge and radius, students can predict how different elements will interact with each other when combined. This allows students to make predictions about how elements will react with each other in more complex compounds, as well as compounds found in nature.
Finally, understanding simple binary ionic compounds helps students analyze the properties of different molecules and compounds. By understanding the characteristics of different ions, students can gain insight into the chemical properties of different compounds. This knowledge can then be used to investigate the behavior of more complex compounds and how they interact with their environment.
In conclusion, understanding simple binary ionic compounds is an important skill for chemistry students to have. By understanding how these compounds are formed, how they interact with each other, and the properties of different ions, students will be better equipped to analyze more complex compounds and compounds found in nature.
Conclusion
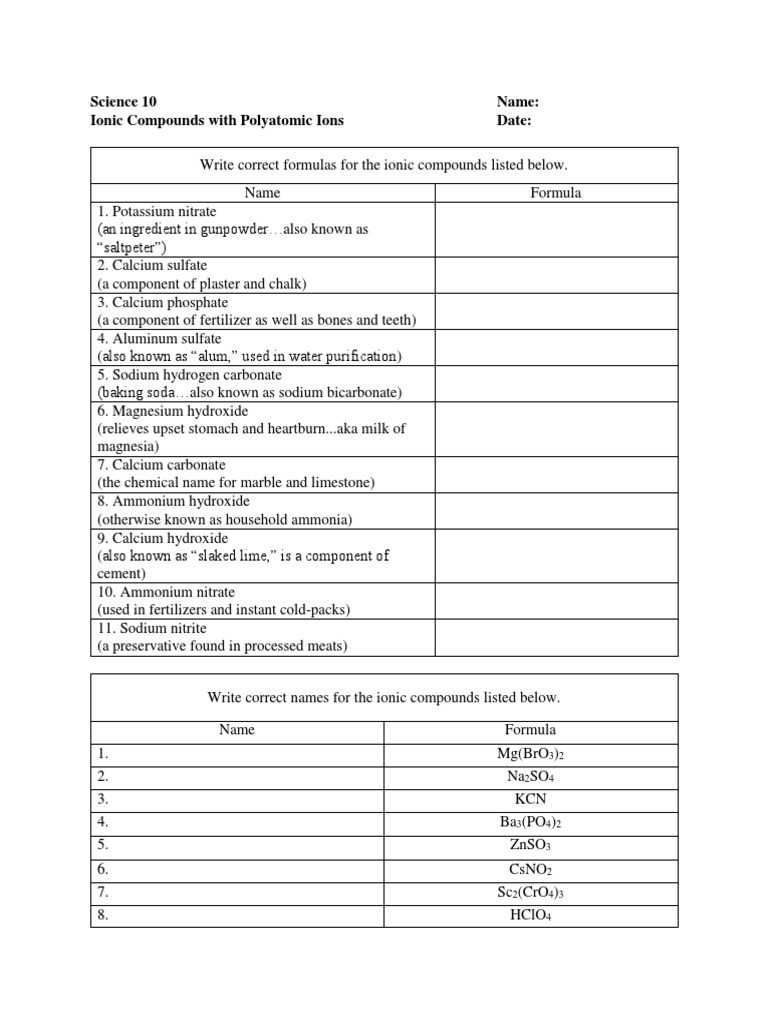
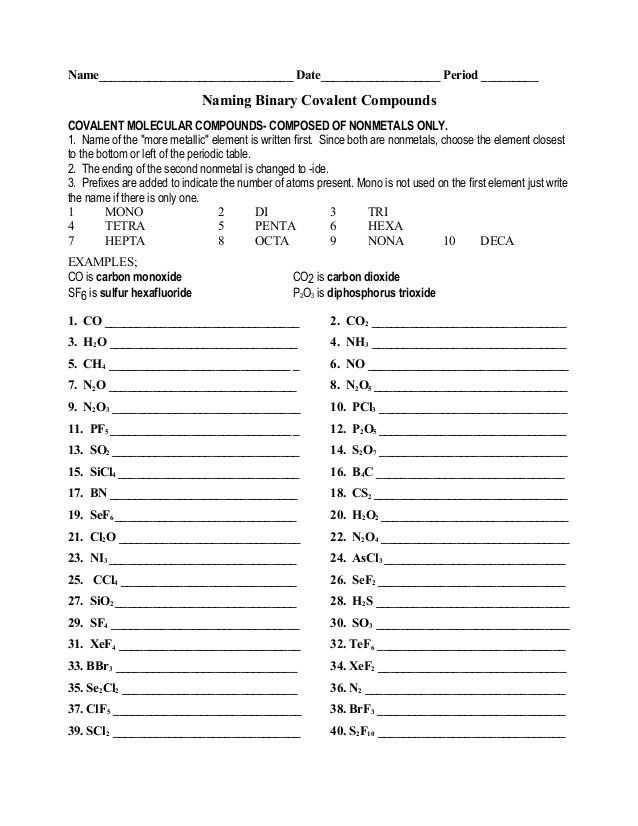
The Simple Binary Ionic Compounds Worksheet is a great way for students to learn about different types of binary ionic compounds and how to write their formulas. Through this worksheet, students can gain a better understanding of how to properly name and write formulas for these compounds. Additionally, the worksheet provides an opportunity to practice and apply the skills they have learned. By completing the worksheet, students can become more confident in their knowledge of binary ionic compounds.
[addtoany]