Exploring the Basics of Quantum Numbers: A Guide to Understanding Quantum Numbers Practice Worksheets
Quantum numbers are an essential tool for the study of atomic and subatomic particles. These numbers provide information about the energy levels, angular momentum, spin, and other properties of a particle. They are used to describe and predict the behavior of particles in a variety of situations. Understanding quantum numbers is essential for anyone studying chemistry, physics, and other related sciences.
This guide provides an overview of quantum numbers and how they are used in practice. It begins with a description of the different types of quantum numbers and their significance. It then provides an explanation of the meaning of each quantum number and how they interact with each other. Finally, this guide includes a number of practice worksheets to help students become familiar with quantum numbers and how they are used.
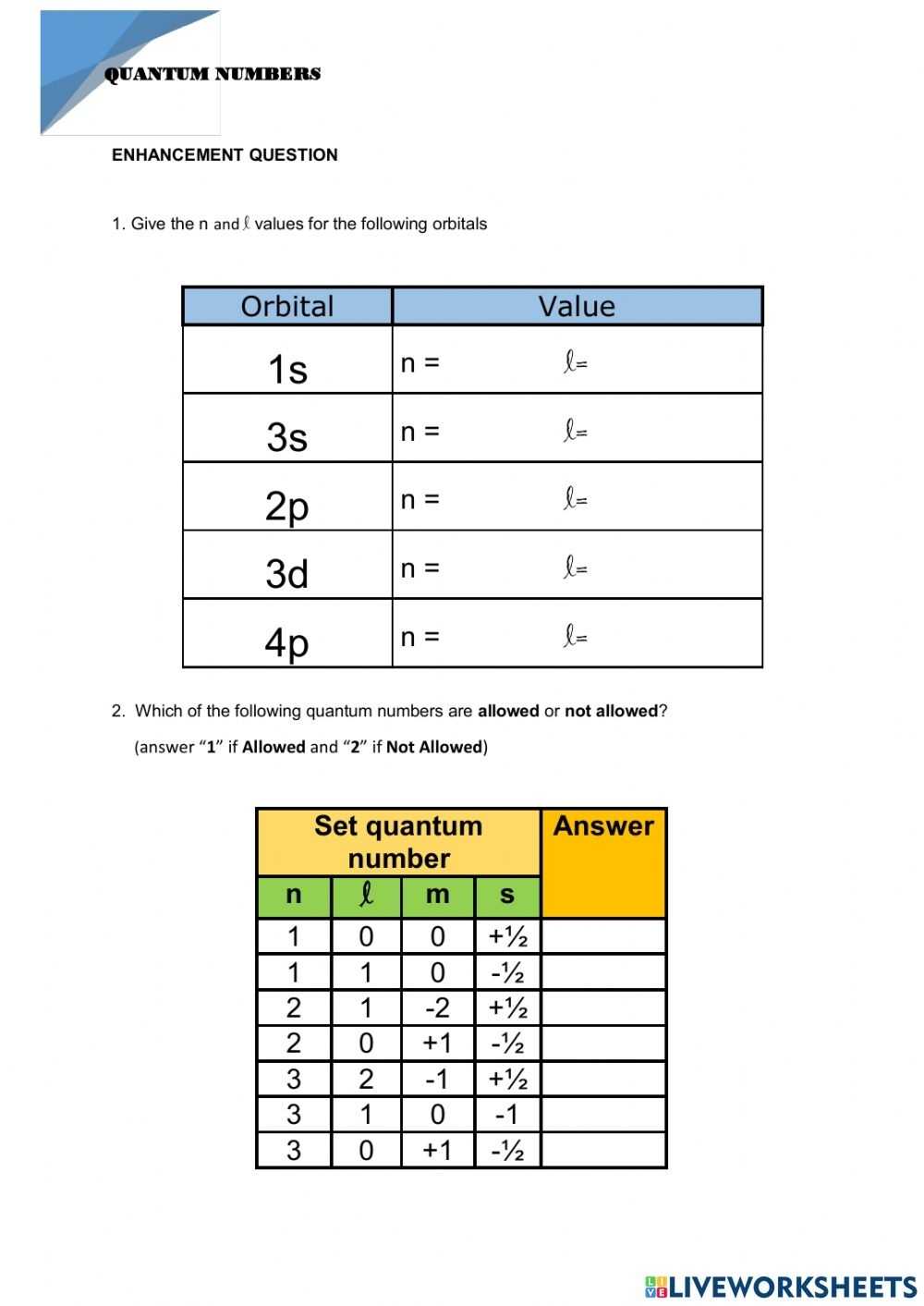
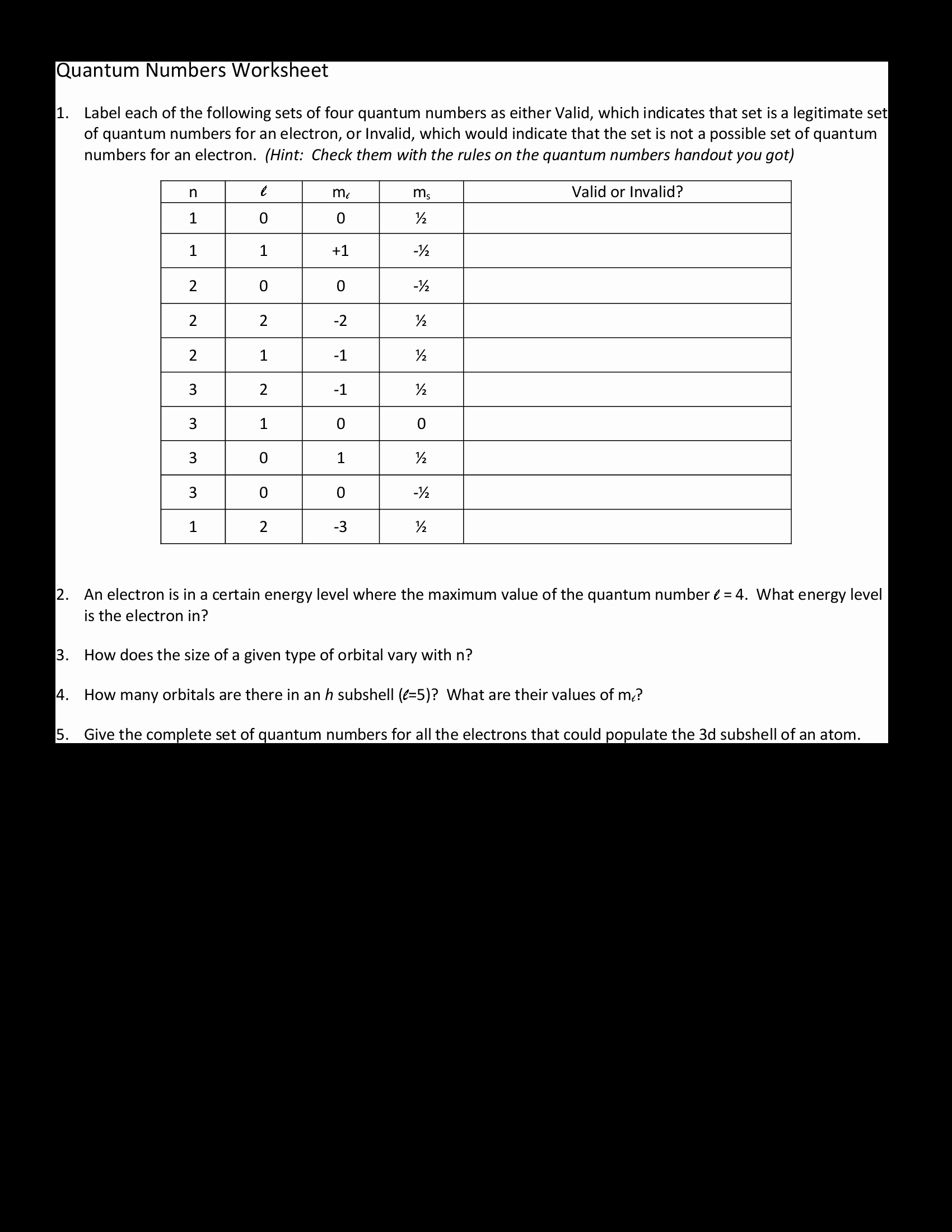
The four main types of quantum numbers are the principal quantum number (n), angular momentum quantum number (l), magnetic quantum number (m_l), and spin quantum number (m_s). The principal quantum number (n) describes the energy level of the electron, while the angular momentum quantum number (l) describes its angular momentum. The magnetic quantum number (m_l) describes the orientation of the angular momentum, and the spin quantum number (m_s) describes the intrinsic spin of the electron.
[toc]
The principal quantum number (n) is always a positive integer and describes the energy level of the electron. It is related to the size of the electron orbit, which determines the amount of energy the electron has. Generally, the higher the principal quantum number, the higher the energy of the electron.
The angular momentum quantum number (l) describes the shape of the electron orbit. It is related to the amount of angular momentum the electron has. This number can be 0, 1, 2, or 3, with 0 representing a spherically symmetric orbital, and 1, 2, and 3 representing an angular momentum of 1, 2, and 3, respectively.
The magnetic quantum number (m_l) is related to the orientation of the angular momentum. This number can range from -l to +l, where l is the angular momentum quantum number. This means that if the angular momentum quantum number is 1 (l = 1), then the magnetic quantum number can range from -1 to +1.
The spin quantum number (m_s) describes the intrinsic spin of the electron. It is related to the direction of the electron’s spin and can be +1/2 or -1/2.
Once the four quantum numbers are known, it is possible to calculate the properties of the electron, such as its energy and angular momentum. A number of practice worksheets are available to help students become familiar with the different types of quantum numbers and how they interact with each other. These worksheets can be used to help students understand how to calculate the different properties of an electron and how the quantum numbers interact with each other.
This guide provides an overview of quantum numbers and how they are used in practice. Understanding the different types of quantum numbers and how they interact with each other is essential for anyone studying physics, chemistry, or other related sciences. The practice worksheets provided in this guide can be used to help students become familiar with the different types of quantum numbers and how they interact with each other.
Quantum Numbers Practice Worksheets: A Comprehensive Guide to Mastering Quantum Numbers
Quantum numbers are an important part of mastering quantum physics. They are used to describe the behavior of particles in an atom and help students understand the behavior of particles in a variety of situations. To become more comfortable with quantum numbers, it is important to practice and become familiar with the different types and how they are used.
This worksheet provides a comprehensive guide to mastering quantum numbers, starting with an in-depth explanation of the four types of quantum numbers, followed by practice questions to test your understanding.
The four types of quantum numbers are principal, angular momentum, magnetic, and spin. The principal quantum number (n) is used to describe the energy level and distance of an electron from the nucleus. The angular momentum quantum number (l) is used to describe the shape of an orbital, such as s, p, d, or f. The magnetic quantum number (ml) is used to describe the orientation of an orbital in space. Lastly, the spin quantum number (ms) is used to describe the spin of an electron.
The workbook contains eight practice questions designed to test your understanding of the four types of quantum numbers. The questions start off with basic questions such as “What is the principal quantum number?” and progress to more complicated questions such as “What is the relationship between the angular momentum quantum number and the shape of an orbital?”
In addition to the practice questions, the workbook also contains an answer key for each question. This allows students to check their answers and make sure they have fully understood the material.
This workbook is an invaluable resource for anyone looking to become more familiar with quantum numbers and the behavior of particles in an atom. With the help of this comprehensive guide and practice worksheets, students will be able to confidently answer any questions regarding quantum numbers and be well on their way to mastering quantum physics.
Quantum Mechanics Demystified: A Step-by-Step Guide to Solving Quantum Numbers Practice Worksheets
Quantum Mechanics Demystified is a step-by-step guide to solving quantum numbers practice worksheets. It is designed to help students tackle the complex concepts involved in quantum physics and guide them through the calculations needed to arrive at the correct answers.
The book begins by introducing the basic concepts of quantum mechanics and its mathematical foundations. It then moves on to explain the different parts of the quantum number equation and how they can be used to solve worksheets. It also covers topics such as wave-particle duality and the Heisenberg uncertainty principle.
The book contains numerous practice worksheets, with solutions and explanations given for each one. Each worksheet is designed to test the student’s understanding of the theory and provide them with the opportunity to practice the calculations required to solve the worksheets.
The book is written in a formal tone, making it easy to follow and understand. Its descriptive writing style makes it a great resource for students of all levels. It is also suitable for those who are new to the subject, providing a comprehensive introduction to the principles of quantum mechanics.
Quantum Mechanics Demystified is an invaluable resource for those studying quantum physics. It is a great way to gain a deeper understanding of the subject and apply the concepts to real-world problems.
Unpacking the Complexity of Quantum Numbers: How to Effectively Utilize Quantum Numbers Practice Worksheets
Quantum numbers are a crucial concept in understanding the behavior of atoms and subatomic particles. They are used to describe the physical and chemical properties of these particles, and the practice of quantum numbers is essential for students of physics and chemistry. Although quantum numbers can be confusing, there are many practice worksheets available to help students master the concept.
To effectively utilize quantum numbers practice worksheets, students should start by familiarizing themselves with the basic components of quantum numbers. Quantum numbers are composed of four distinct pieces of information: the principal quantum number (n), the angular momentum quantum number (ℓ), the magnetic quantum number (mℓ), and the spin quantum number (ms). Each of these numbers has a specific meaning and can be used to calculate the energy, angular momentum, and other properties of electrons.
Once students are familiar with the components of quantum numbers, they should begin with simple practice worksheets. Such worksheets will typically provide diagrams or tables of quantum numbers for different atoms or subatomic particles. By filling out the diagrams or tables with the appropriate quantum numbers, students can gain a better understanding of how the numbers relate to each other.
Students can also use practice worksheets to compare the properties of different atoms or particles. This can be done by comparing the quantum numbers of different particles and examining how their energy levels and angular momentum change. Such a comparison can help students understand the effects of different quantum numbers on the behavior of atoms and particles.
Finally, students should use practice worksheets to better understand the relationships between quantum numbers and other physical properties. For example, by plotting a graph of the energy levels of different particles and noting the corresponding quantum numbers, students can gain a better understanding of the relationship between energy and quantum numbers. This understanding can be used to predict the behavior of atoms and particles in different situations.
By utilizing quantum numbers practice worksheets, students can gain a better understanding of the complex concept of quantum numbers. Through practicing with these worksheets, students can better understand the physical and chemical properties of atoms and particles, and how the quantum numbers can be used to calculate their properties. With this knowledge, students can make better predictions about the behavior of atoms and particles in different situations.
Conclusion
In conclusion, the Quantum Numbers Practice Worksheet is a great resource for learning about the essential concepts of quantum numbers. It helps students to understand the fundamental concepts of energy levels, angular momentum, and spin. It also provides numerous examples to help students grasp the principles of quantum numbers. With the help of this worksheet, students can gain a better understanding of the fundamentals of quantum theory, which can help them in their daily lives.
[addtoany]