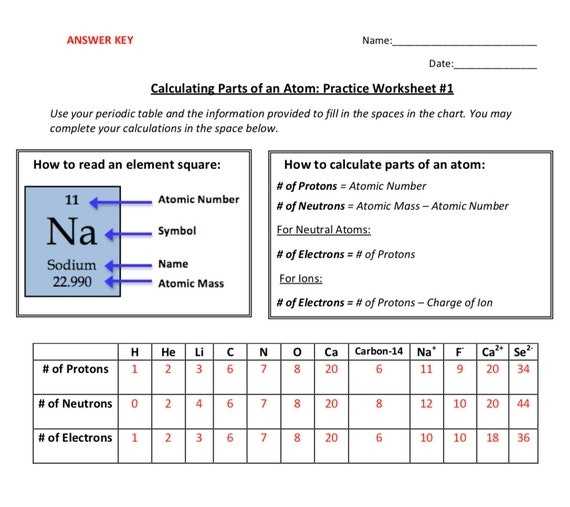
Exploring the Basics of Protons Neutrons and Electrons with a Worksheet
Protons, neutrons, and electrons are the building blocks of atoms, the basic units of matter. They are the fundamental components of chemistry and physics, and understanding how they interact is essential to comprehending the physical and chemical properties of matter. This worksheet will help you explore the basics of protons, neutrons, and electrons so that you can gain a better understanding of the fundamental principles of chemistry and physics.
Proton:
A proton is a small, positively charged particle that is found in the nucleus of an atom. It has a mass of 1.673 x 10-24 grams, and it is composed of two up quarks and one down quark. Protons are the main component of the atomic nucleus and are responsible for the chemical properties of atoms.
Neutron:
A neutron is a neutral, uncharged particle that is found in the nucleus of an atom. It has a mass of 1.675 x 10-24 grams and is composed of two down quarks and one up quark. Neutrons add mass to the nucleus and can interact with other particles through the strong nuclear force.
[toc]
Electron:
An electron is a small, negatively charged particle that orbits the nucleus of an atom. It has a mass of 9.109 x 10-28 grams and is composed of a single down quark. Electrons are responsible for the electrical properties of atoms and interact with other particles through the electromagnetic force.
These three particles make up the basic components of an atom. Protons and neutrons form the nucleus, while electrons orbit the nucleus. When protons and neutrons combine, they form the nucleus of an atom, which determines the element. By understanding the basics of protons, neutrons, and electrons, you can gain a better understanding of how atoms interact and how they form chemical bonds with one another.
Investigating the Properties of Protons Neutrons and Electrons with a Worksheet
Investigating the Properties of Protons, Neutrons, and Electrons
The atomic structure of our universe is composed of three distinct particles: protons, neutrons, and electrons. Each has its own fundamental properties, which interact in complex ways to form the elements of the periodic table. This worksheet will explore the properties of these particles and how they relate to one another.
Protons
Protons are positively charged particles found in the nucleus of an atom. They are the heaviest of the three particles and are responsible for most of the mass of an atom. Protons have a charge of +1, and the number of protons in an atom determines its atomic number.
Neutrons
Neutrons, like protons, are found in the nucleus of an atom. They have no charge, and are slightly heavier than protons. Neutrons are responsible for the stability of the atom, as they help to balance the positive charges of the protons. The number of neutrons in an atom is known as its neutron number.
Electrons
Electrons are negatively charged particles that orbit the nucleus of an atom. They are much lighter than protons and neutrons, and their charge is -1. The number of electrons in an atom is equal to the number of protons, so the overall charge of an atom is neutral.
Relationships Among Protons, Neutrons, and Electrons
Protons and neutrons form the nucleus of an atom, while electrons orbit the nucleus in shells. The number of protons in an atom determines its atomic number, while the number of neutrons determines its neutron number. The number of electrons in an atom is equal to the number of protons, so the overall charge of an atom is neutral.
Conclusion
This worksheet has explored the fundamental properties of protons, neutrons, and electrons, as well as their relationships to one another. By understanding these particles and the ways in which they interact, we can gain a better understanding of the elements of the periodic table and the structure of atoms.
Understanding the Interaction between Protons Neutrons and Electrons with a Worksheet
The interaction between protons, neutrons, and electrons is an essential part of understanding atomic structure. In order to gain a better understanding of this interaction, a worksheet can provide a useful tool for exploring the different aspects of the relationship between the three particles.
The worksheet should begin by providing a brief overview of the atomic structure. This should include the definition of each particle, as well as a description of their properties. This overview should also include a description of the strong and weak nuclear forces, which are responsible for holding protons and neutrons together to form the nucleus of an atom.
Once the basic information has been presented, the worksheet should then move on to provide an explanation of the interaction between protons, neutrons, and electrons. This should include a discussion of how the particles interact through the exchange of particles and how this affects the stability of the atom. The worksheet should also explain how the particles can interact to form molecules, as well as how the particles can interact to form different types of compounds.
The worksheet should then move on to explore the different ways in which the particles interact with each other. This could include a discussion of the quantum mechanical behavior of the particles, as well as a description of the processes of nuclear fission and fusion. This section should also include a discussion of how the particles interact with energy, such as through radiation and heat.
Finally, the worksheet should conclude with a discussion of the role of the electron in the atomic structure. This should include a description of the electrons’ ability to move around the nucleus and its ability to absorb and release energy. This section should also include a discussion of the role of the electron in forming bonds between atoms and molecules.
By providing a thorough description of the interaction between protons, neutrons, and electrons, a worksheet can serve as an effective tool for gaining an understanding of the atomic structure. Through the use of this worksheet, students can gain a better understanding of the relationship between the particles, as well as how they interact with each other.
Developing Problem-Solving Skills using a Protons Neutrons and Electrons Worksheet
Developing problem-solving skills is an essential part of any educational journey. In order to better understand the world around us, it is important to understand the basic principles of the building blocks of matter. One way to help students develop their problem-solving skills is by using a protons, neutrons and electrons worksheet.
Such a worksheet can provide students with a hands-on approach to understanding the properties of atoms and their subatomic particles. It can help them understand the basic structure and function of matter, as well as the concept of atomic weight, which is a measure of the number of protons, neutrons and electrons in an atom. Understanding the relative weights of these particles can help students better understand the behavior of matter and how it reacts to different forces.
The worksheet should also provide students with a visual representation of the structure of atoms, as well as their relative sizes. This can help students visualize the differences between different types of atoms, as well as how they interact with each other. By looking at the diagram, students can also gain an understanding of how the protons, neutrons and electrons interact with each other to form different types of molecules.
Once a student has a grasp of the basic principles of the protons, neutrons and electrons worksheet, they can then use it to help them solve problems. For example, they can use the worksheet to calculate the number of protons and neutrons in an atom, and then use this knowledge to determine the mass of an atom. This can help them understand how different elements react to different forces, such as attraction and repulsion.
Using a protons, neutrons and electrons worksheet can also help students develop their problem-solving skills. By understanding the structure and behavior of atoms, they can use the worksheet to determine the properties of different molecules and their reactions with each other. This can help them develop a better understanding of how different elements interact with each other and how they can be manipulated to create different compounds.
By using a protons, neutrons and electrons worksheet, students can increase their problem-solving abilities, making them better able to identify and solve complex problems. This can help them succeed in their studies and in their future endeavors.
Conclusion
In conclusion, the Protons Neutrons And Electrons Worksheet is an excellent resource for students to learn about the structure of atoms and how they interact with each other. This worksheet helps students learn the basics of atomic structure and how protons, neutrons, and electrons work together to form atoms. By understanding the basics of atomic structure, students can better understand the physical and chemical properties of matter and how they interact with each other.
[addtoany]