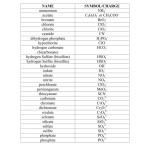
Understanding How to Use Nomenclature Worksheets to Name Monatomic Ions
Nomenclature worksheets are an essential tool for anyone looking to name monatomic ions, or ions made up of a single atom. Monatomic ions are either positively or negatively charged particles, and they can be either an atom or a group of atoms. Naming these ions correctly is essential for understanding the science behind them and for communicating accurately about them.
Nomenclature worksheets can be used to name monatomic ions in a variety of ways. The most common method is to use the charge of the ion to determine its name. For a positive ion, the name is the element plus the suffix “ium”. For a negative ion, the name is the element plus the suffix “ide”. For example, a positive ion of sodium would be named “sodiumium” and a negative ion of sodium would be named “sodiumide”.
In addition to the charge of the ion, nomenclature worksheets can also be used to name monatomic ions based on their chemical symbol. For example, a positive ion of carbon would be named “carbonium” and a negative ion of carbon would be “carbide”. Similarly, a positive ion of oxygen would be named “oxygenium” and a negative ion of oxygen would be “oxide”.
[toc]
Finally, nomenclature worksheets can also be used to name monatomic ions based on their molecular mass. For example, a positive ion of potassium would be named “potassiumium” and a negative ion of potassium would be “potassiumide”. The same is true for any other element, as well as for molecules made up of multiple atoms.
Nomenclature worksheets are an invaluable tool for anyone looking to name monatomic ions accurately. By understanding the principles behind the different methods of naming ions, it is possible to quickly and accurately identify the correct name for each ion.
Exploring the Different Types of Monatomic Ions and Their Nomenclature
Monatomic ions are single atoms that have either gained or lost electrons, resulting in them having a net electrical charge. Monatomic ions are classified by their charge, which can either be positive or negative. This charge is determined by the number of protons versus electrons. Positive monatomic ions, or cations, have lost electrons, resulting in a net positive charge. On the other hand, negative monatomic ions, or anions, have gained electrons, resulting in a net negative charge.
Nomenclature for monatomic ions is based on the name of the element the ion is derived from. For cations, the name of the element will remain the same, but the ending of the name will change depending on the charge of the cation. For example, if an element has a +1 charge, the element will end in “-ium”, such as sodium (Na+) becoming sodium ion (Na+1). If the cation has a +2 charge, the element will end in “-ide”, such as magnesium (Mg2+) becoming magnesium ion (Mg2+).
Anions also retain the name of the element they are derived from, but the ending of the name changes depending on the charge of the anion. For example, if an element has a -1 charge, the element will end in “-ide”, such as chlorine (Cl-) becoming chloride ion (Cl-1). If the anion has a -2 charge, the element will end in “-ate”, such as sulfur (S2-) becoming sulfate ion (S2-).
In summary, monatomic ions are single atoms that have either gained or lost electrons, resulting in them having a net electrical charge. This charge is determined by the number of protons versus electrons. Positive monatomic ions, or cations, have lost electrons and negative monatomic ions, or anions, have gained electrons. For cations, the name of the element will end in “-ium” or “-ide” depending on their charge. For anions, the name of the element will end in “-ide” or “-ate” depending on their charge.
Unraveling the Rules of Nomenclature Worksheets to Name Monatomic Ions
Investigating the Properties of Monatomic Ions and Their Nomenclature
Conclusion
The Nomenclature Worksheet 1 Monatomic Ions provides a useful overview of the different kinds of monatomic ions and how to name them. It also gives us an insight into the different charges of ions and how they can be represented. Understanding the nomenclature of monatomic ions is essential for understanding chemical reactions. With this knowledge, chemists can effectively identify compounds and substances and how they interact with each other.
[addtoany]