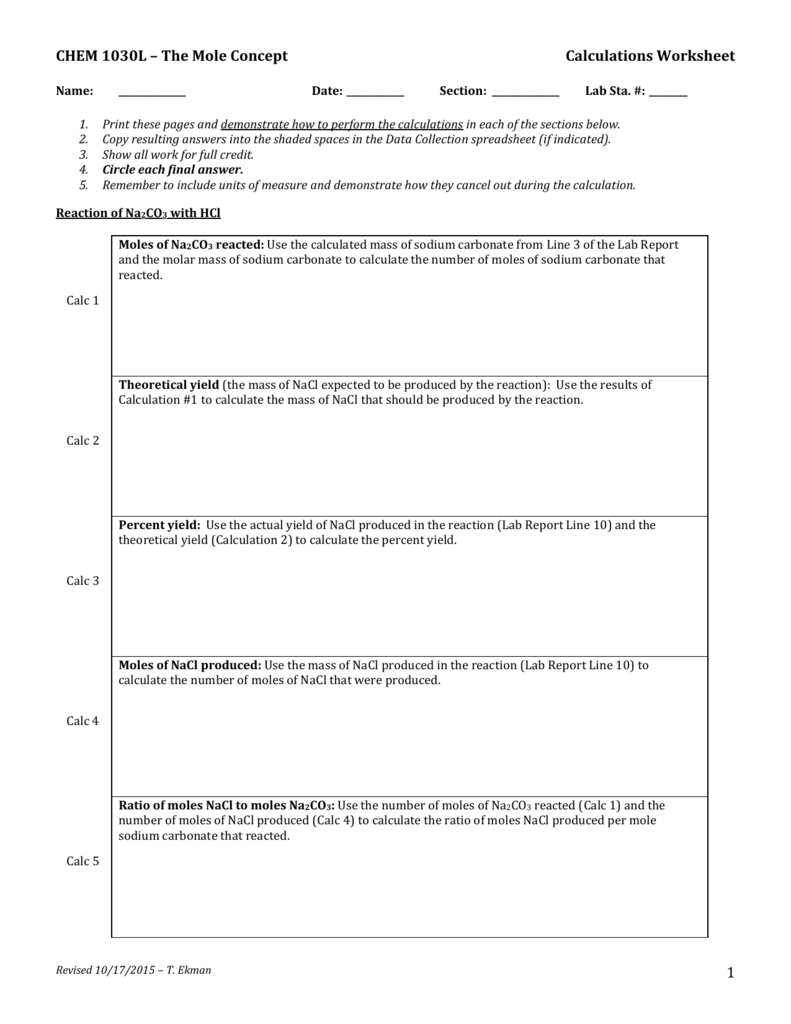
Types and Uses of Mole Calculations
Mole calculations are used to determine the amount of a substance in a given mass, volume, or concentration. This type of calculation is especially important in chemistry, where the number of moles of different substances is crucial in defining the properties of a chemical reaction. The calculations are based on the Avogadro constant, which states that there are 6.02 x 1023 particles per mole of any substance.
Mole calculations can be used to determine the mass of a substance in grams, the volume of a substance in liters, or the concentration of a substance in molarity. For example, if a chemist needs to determine the mass of a given number of moles of a substance, they can use mole calculations to convert from moles to grams. Similarly, if a chemist needs to determine the volume of a given number of moles of a substance, they can use mole calculations to convert from moles to liters. Finally, if a chemist needs to determine the concentration of a substance in molarity, they can use mole calculations to calculate the moles of the substance per liter.
Mole calculations are also used to determine the number of moles of a given substance in a given mass, volume, or concentration. For example, if a chemist needs to determine the number of moles of a given substance in a given mass, they can use mole calculations to convert from the mass to moles. Similarly, if a chemist needs to determine the number of moles of a given substance in a given volume, they can use mole calculations to convert from the volume to moles. Finally, if a chemist needs to determine the number of moles of a given substance in a given concentration, they can use mole calculations to convert from the concentration to moles.
[toc]
Overall, mole calculations are an essential tool for chemists and scientists alike. They allow for the accurate and precise determination of the amount of a substance in a given mass, volume, or concentration, and can be used to determine the number of moles of a given substance in a given mass, volume, or concentration.
Understanding the Basic Principles of Mole Calculations
Mole calculations are a fundamental concept in chemistry, and are essential for understanding chemical reactions and analyzing the composition of matter. The mole is a unit of measurement used to describe the amount of a substance, and is defined as the amount of a substance that contains an Avogadro’s number of particles. The Avogadro’s number, which is equal to 6.022×1023, is a constant that is used in a variety of calculations.
Mole calculations involve multiplying the number of moles of a substance by its molecular weight. The molecular weight is the sum of the atomic weights of all the atoms in a molecule. This is an important concept to understand as it is used to determine the amount of a substance needed to react with another substance. For example, if the molecular weight of a substance is 40 g/mol and the amount of moles needed is 2, then the amount of the substance needed is 80 g.
Another important concept in mole calculations is the concept of molar mass. This is the mass of one mole of a substance. Molar mass is calculated by multiplying the number of moles of a substance by its molecular weight. For example, if the molecular weight of a substance is 40 g/mol and the amount of moles needed is 2, then the molar mass of the substance is 80 g/mol.
Mole calculations are also used to calculate the concentration of a solution. This is done by dividing the number of moles of a substance by the total volume of the solution. This is an important tool for determining the amount of a substance needed to produce a certain reaction in a solution.
Mole calculations are an essential part of understanding chemical reactions and analyzing the composition of matter. By understanding the basic principles of moles and their calculations, chemists can accurately determine the amount of a substance needed to produce a certain reaction in a solution and analyze the composition of matter.
How Mole Calculations Help in Chemistry
Mole calculations are a critical component of chemistry that allow for the accurate measurement of matter. By calculating the number of moles of a substance, chemists can accurately determine the amount of a given material present in a system. This information is essential for accurately assessing the nature of the reaction taking place.
Mole calculations begin with the mole ratio, which is the ratio of the number of moles of each reactant and product. This ratio can then be used to determine the amount of each reactant and product in a given reaction. This is done by multiplying the mole ratio by the total number of moles of each reactant or product.
Mole calculations can also be used to determine the amount of energy released or absorbed in a reaction. The energy per mole is determined by dividing the total amount of energy released or absorbed by the number of moles of each reactant or product. This information allows chemists to accurately assess the nature of the reaction taking place.
Finally, mole calculations can be used to determine the amount of heat released or absorbed in a reaction. This is done by multiplying the energy per mole by the number of moles of each reactant or product. This allows chemists to accurately calculate the amount of heat released or absorbed in a reaction.
Overall, mole calculations are an essential component of chemistry that allow chemists to accurately measure and assess the nature of a reaction. By accurately calculating the amount of reactants, products, energy released or absorbed and heat released or absorbed in a reaction, chemists can gain a better understanding of the chemical process taking place.
Common Mistakes to Avoid When Working with Mole Calculations
Mole calculations can be a challenging concept to master and, if not done correctly, can lead to erroneous results. To ensure accurate results, there are several common mistakes to be aware of and avoid when working with mole calculations.
First, it is important to remember that the units of moles must be consistent. For example, if the initial calculation involves grams, the final result should also be expressed in grams. If the units are not consistent, the result will be incorrect.
Second, it is essential to take into account the number of moles in a given sample. Many students make the mistake of assuming that the sample is made up of one mole. However, if the sample contains more than one mole, the calculation should reflect this.
Third, it is important to be aware of the difference between moles and molecules. Moles refer to the number of atoms and molecules refer to the number of molecules. When performing mole calculations, it is crucial to distinguish between the two.
Fourth, it is important to remember that in order to calculate the molar mass of a compound, all of its constituent elements must be taken into consideration. For example, if a compound contains three different elements, the molar mass of each element must be multiplied by the number of atoms of each element in the compound.
Finally, it is important to remember that the units used in the calculation must be consistent. For example, if the initial calculation is done in milligrams, the final result should also be expressed in milligrams. If the units are not consistent, the result will be incorrect.
By avoiding these common mistakes, it is possible to ensure accurate results when working with mole calculations.
Conclusion
The Mole Worksheet 1 is a great resource for students to practice their understanding of the mole concept. It provides a variety of exercises that allow students to practice the mole concept in multiple ways. It also provides students with an opportunity to apply their knowledge of the mole concept to real-world problems. With the help of this worksheet, students can gain a better understanding of the mole concept and be better prepared for future academic and professional pursuits.
[addtoany]