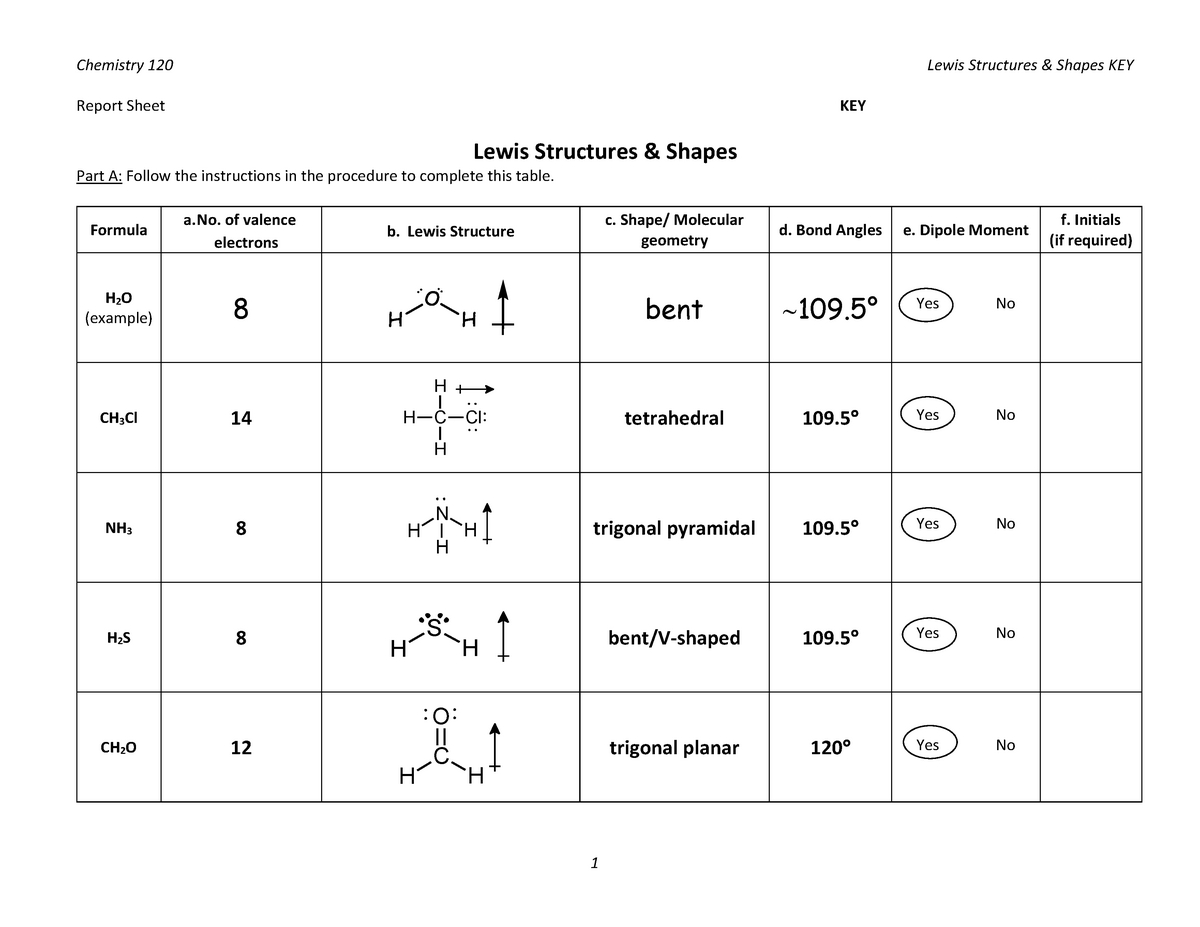
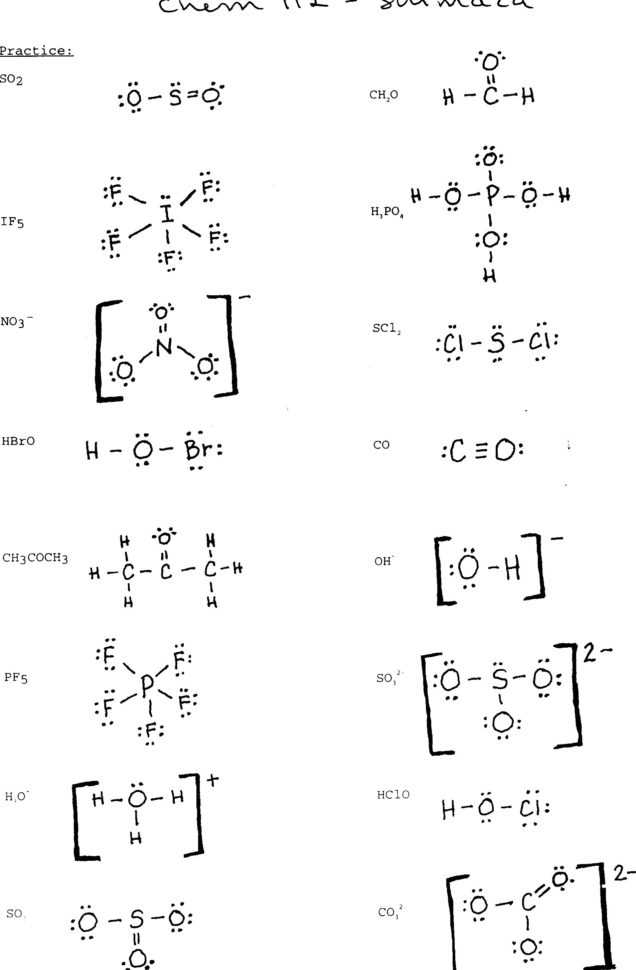
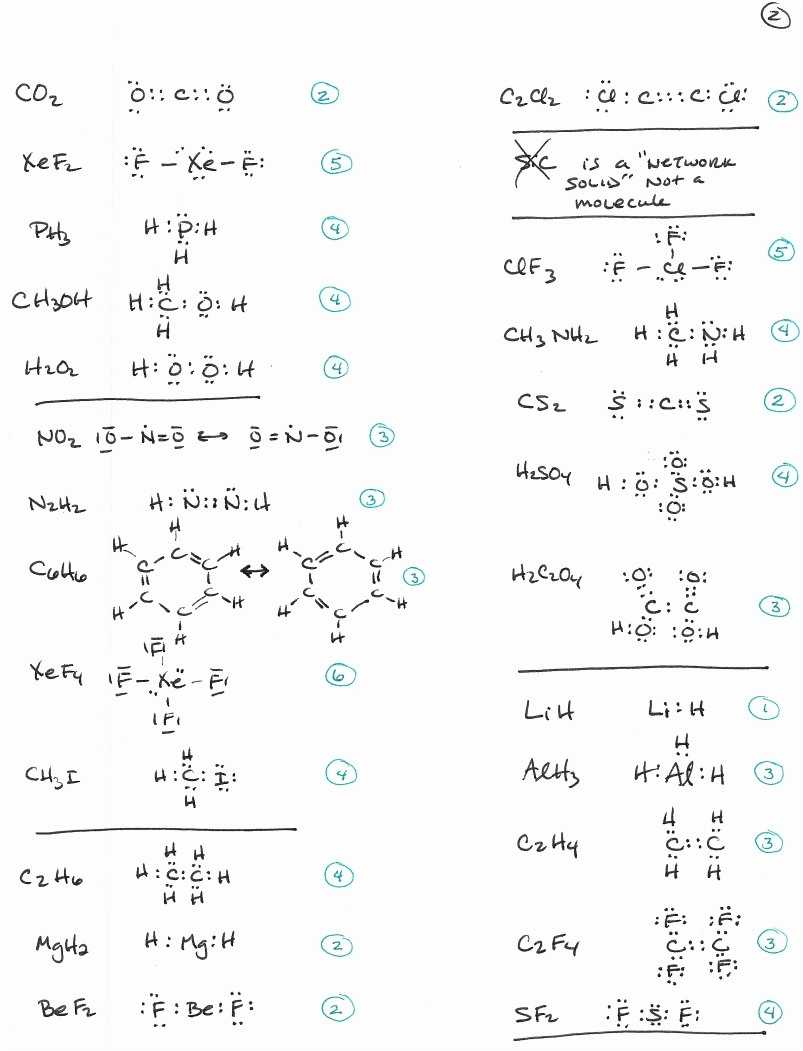
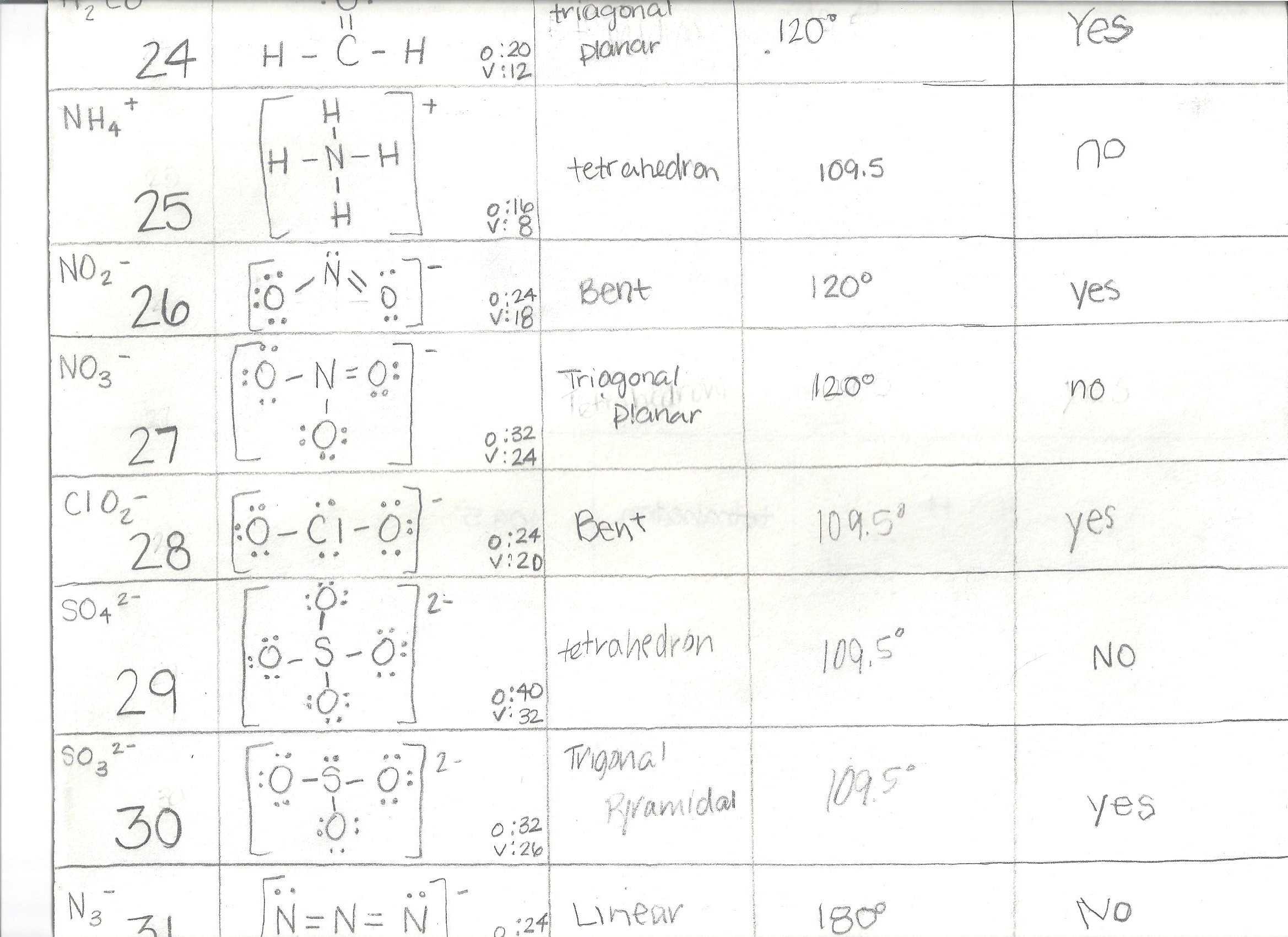
Utilizing Lewis Structures Worksheets with Answers to Aid in Chemistry Problem-Solving
Lewis structures worksheets with answers are a valuable resource for students attempting to solve chemistry problems. These worksheets provide a simple and effective way to introduce students to the basics of atomic structure and chemical bonding. Students are required to draw and interpret Lewis diagrams, which are diagrams that represent the outermost electrons of an atom. By analyzing the diagrams, students can identify the types of bonds present in a molecule and determine the number of valence electrons that an atom has.
The worksheets provide step-by-step instructions for students to draw Lewis diagrams and interpret the information that they represent. Furthermore, the worksheets also provide answers to common questions about chemical bonding and atomic structure. For example, students can learn the number of valence electrons present in an atom, the types of bonds formed between atoms, and the stability of molecules. The answers to the questions are accompanied by clear and concise explanations so that students can gain a better understanding of the concepts.
The worksheets also provide a great way for students to practice problem-solving skills. By providing students with different structures to analyze, they can hone their skills in interpreting diagrams and solving chemistry problems. Furthermore, the worksheets provide an opportunity for students to compare and contrast different structures, allowing them to gain a deeper understanding of chemical bonding and atomic structure.
[toc]
Overall, Lewis structures worksheets with answers are an invaluable resource for any student studying chemistry. By providing students with step-by-step instructions and answers to common questions, students can gain a better understanding of the basics of atomic structure and chemical bonding. Furthermore, the worksheets provide an excellent way for students to practice problem-solving skills. Thus, utilizing Lewis structures worksheets with answers can be a great aid in helping students to hone their chemistry problem-solving abilities.
Exploring the Basics of Lewis Structures with a Step-by-Step Worksheet and Answer Key
A Lewis structure is an understanding of chemical bonding that helps to explain the structure of a molecule. It is a visual representation of the electrons around a central atom in a molecule, and is an important concept for understanding the behavior of molecules. In order to draw a Lewis structure, there are several steps that must be followed. This worksheet will provide a step-by-step guide to drawing Lewis structures, and will include an answer key to check your work.
Step 1: Count the total number of valence electrons in the molecule. Valence electrons are the electrons located in the outermost shell of an atom and determine the chemical behavior of the atom. All atoms in the molecule will contribute their valence electrons to the Lewis structure.
Step 2: Place the central atom in the middle of the structure. This atom is typically the least electronegative atom in the molecule, and is surrounded by the other atoms.
Step 3: Connect the atoms in the molecule with single bonds. A single bond is formed when two atoms share two electrons. Each bond should contain one electron from each atom.
Step 4: Add lone pairs to the central atom until all of the valence electrons have been used. Lone pairs are pairs of electrons that are not shared with another atom.
Step 5: Assign formal charges to each atom. Formal charges are the differences between the number of valence electrons that the atom has in its neutral form and the number of electrons assigned to the atom in the Lewis structure.
Step 6: Determine the most stable Lewis structure. The most stable Lewis structure will be the one that has the least amount of formal charge.
Answer Key:
1. The total number of valence electrons in the molecule is 20.
2. The central atom is oxygen.
3. The atoms are connected with single bonds.
4. The central atom has six lone pairs.
5. The formal charges are 0 for oxygen, +1 for hydrogen and -1 for chlorine.
6. The most stable Lewis structure is the one with zero formal charges.
How to Effectively Use Lewis Structures Worksheets to Master the Art of Drawing Molecules
Lewis Structures worksheets are a valuable tool for mastering the art of drawing molecules. They provide a framework for drawing structural formulas by providing diagrams of molecules and prompting the student to fill in the details. By working through the worksheet, students will become familiar with the basics of drawing molecules and gain a deeper understanding of their structure.
The first step in using Lewis Structures worksheets is to familiarize yourself with the structure of the molecules and the shapes of the atoms. This can be done by studying diagrams and carefully observing the details. Once this information is understood, students can begin to draw their own molecules by following the instructions provided by the worksheet.
When working through the worksheet, it is important to pay close attention to the instructions given. It is important to understand the purpose of the diagrams, as well as the rules for building and connecting the atoms. Understanding these rules will allow the student to draw their own molecules accurately and with confidence.
Once the student has a grasp of the basics of drawing molecules, they can move on to more complex molecules. By following the instructions provided by the worksheet, it is possible to build more intricate molecules with more complex connections between the atoms. This will help to develop the student’s understanding of the structure of molecules and improve their ability to draw them accurately.
In addition to drawing molecules, Lewis Structures worksheets can also be used to better understand the properties of molecules. By studying diagrams, students can learn how different elements interact with one another and how their properties can affect the properties of the molecule. This will help to better understand the behavior of molecules, as well as the potential uses of molecules in the future.
Students can also use Lewis Structures worksheets to practice drawing molecules in different shapes and sizes. This will help to develop their skills in drawing molecules accurately and with confidence. As students gain more experience drawing molecules, they will be able to draw molecules of increasing complexity with ease.
By using Lewis Structures worksheets, students can quickly become proficient in the art of drawing molecules. With practice and patience, students can gain a deep understanding of the structure of molecules and become experts in the field of molecular drawing. With the help of Lewis Structures worksheets, students can become skilled in the art of drawing molecules and use this skill to their advantage.
Conclusion
The Lewis Structures Worksheet With Answers is an effective tool for teaching students the basics of Lewis Structures. It provides step-by-step instructions on how to draw and interpret Lewis Structures and provides clear explanations of the concepts involved. The worksheet also contains practice questions that help reinforce the concepts and allow students to gain a better understanding of the material. Overall, the Lewis Structures Worksheet With Answers is an excellent resource for teaching Lewis Structures and can be used to help students learn and master this important part of Chemistry.
[addtoany]