Exploring the Benefits of Using Lewis Dot Structure Worksheet Answers
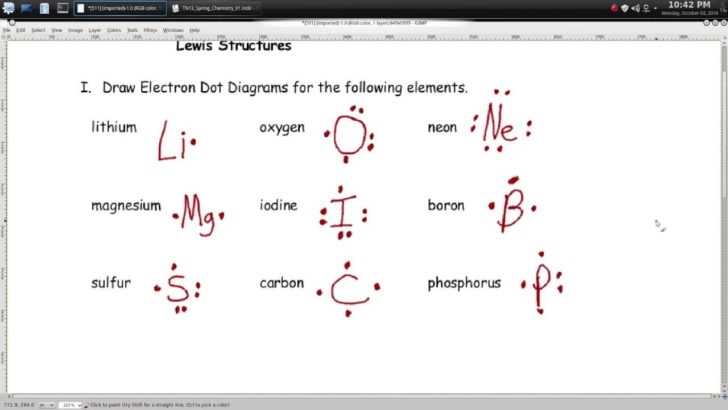
Lewis dot structure worksheets are a useful tool for teaching students about the basics of chemical bonding and structure. By providing students with an organized, easy-to-follow template, these worksheets can help students learn the principles of Lewis dot structures and apply them to their own studies.
When using Lewis dot structure worksheets, students can benefit in a number of ways. First, they provide a visual aid to help students understand the principles of Lewis dot structures. By using the worksheet, students can easily identify elements, draw electron dot diagrams, and determine the types of bonds formed between two atoms. This can be especially helpful when learning about covalent, ionic, and metallic bonds.
Another benefit of using Lewis dot structure worksheets is that they can help students understand the implications of the structure. By providing students with the answers to their questions, they can gain a better understanding of the implications of their structure. For example, students can learn about the types of electron pair repulsions that occur when two atoms are bonded together and the types of forces that bind them together.
[toc]
Finally, Lewis dot structure worksheets can be used to evaluate the accuracy of students’ work. By providing students with a correct answer key, they can check their work to make sure that their calculations are correct. This can be especially helpful when working on more complex structures, such as those involving multiple atoms and multiple bonds.
Overall, Lewis dot structure worksheets are a helpful tool for teaching students about the basics of chemical bonding and structure. By providing students with an organized template and the answers to their questions, these worksheets can help students understand the principles of Lewis dot structures and apply them to their own studies. With the help of these worksheets, students can gain a better understanding of the implications of their structure and learn to evaluate the accuracy of their work.
A Comprehensive Guide to Answering Lewis Dot Structure Questions
Lewis dot structures are used to illustrate the arrangement of electrons in molecules. The purpose of a Lewis dot structure is to depict the valence electrons of an atom or molecule in order to better understand its chemical properties. This guide provides a detailed overview of the steps required to answer Lewis dot structure questions.
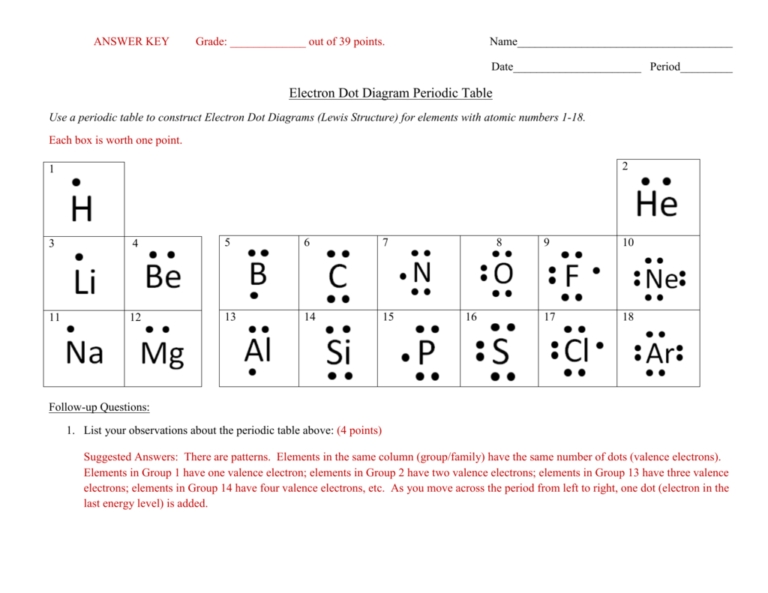
The first step is to determine the total number of valence electrons in the molecule. This is done by looking at the periodic table and adding the valence electrons of each atom in the molecule. It is important to note that noble gases typically do not share electrons and thus do not need to be included when counting valence electrons.
The next step is to arrange the atoms in the molecule in order of their atomic number. This will ensure that the molecule is accurately depicted in the Lewis dot structure.
Once the atoms are arranged, the electrons should be placed in pairs around each atom. Electrons should be placed in a clockwise or counterclockwise direction. It is important to note that the number of electrons placed around each atom should not exceed eight.
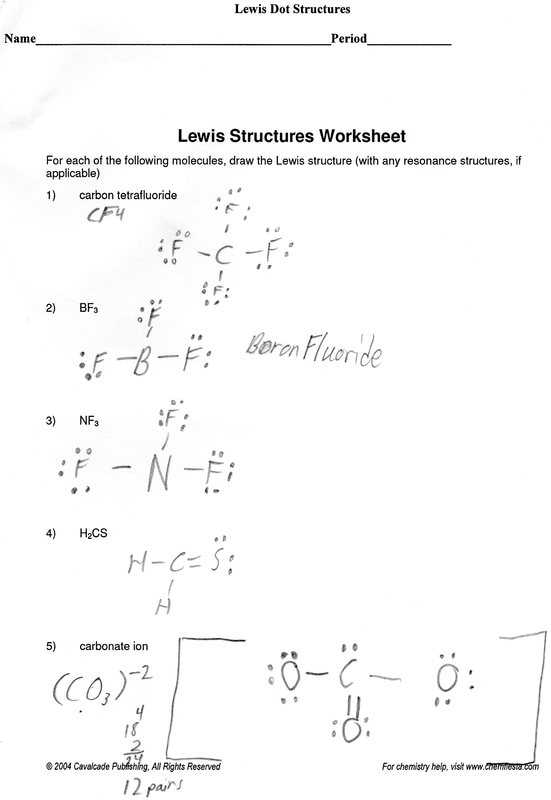
The last step is to draw a single bond between each pair of atoms. This will represent the sharing of electrons between the two atoms. This will complete the Lewis dot structure for the molecule.
Following these steps will allow you to accurately answer Lewis dot structure questions. Understanding how to draw Lewis dot structures is important for understanding the chemistry of a molecule and can be used to predict its properties.
How to Utilize Lewis Dot Structure Worksheet Answers to Improve Your Chemistry Grade
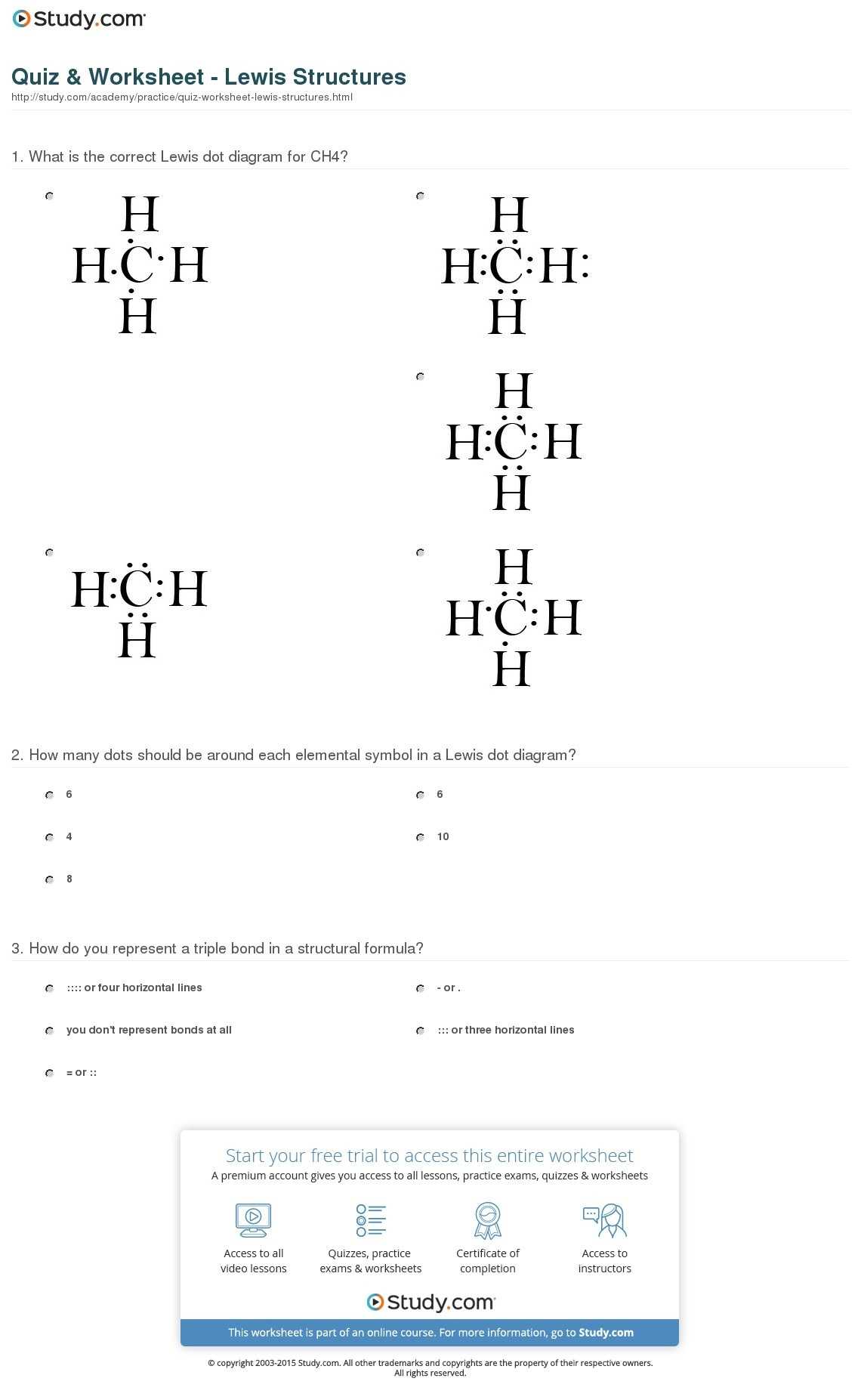
Improving one’s chemistry grade can be a difficult task, but with the help of Lewis Dot Structure Worksheet Answers, students can gain a better understanding of the material and improve their overall grade. Lewis Dot Structure Worksheets contain diagrams of molecules and their constituent atoms, along with the number of valence electrons for each atom. By correctly completing the worksheet, students can determine the type of bond formed between atoms and gain an understanding of the structure of the molecule.
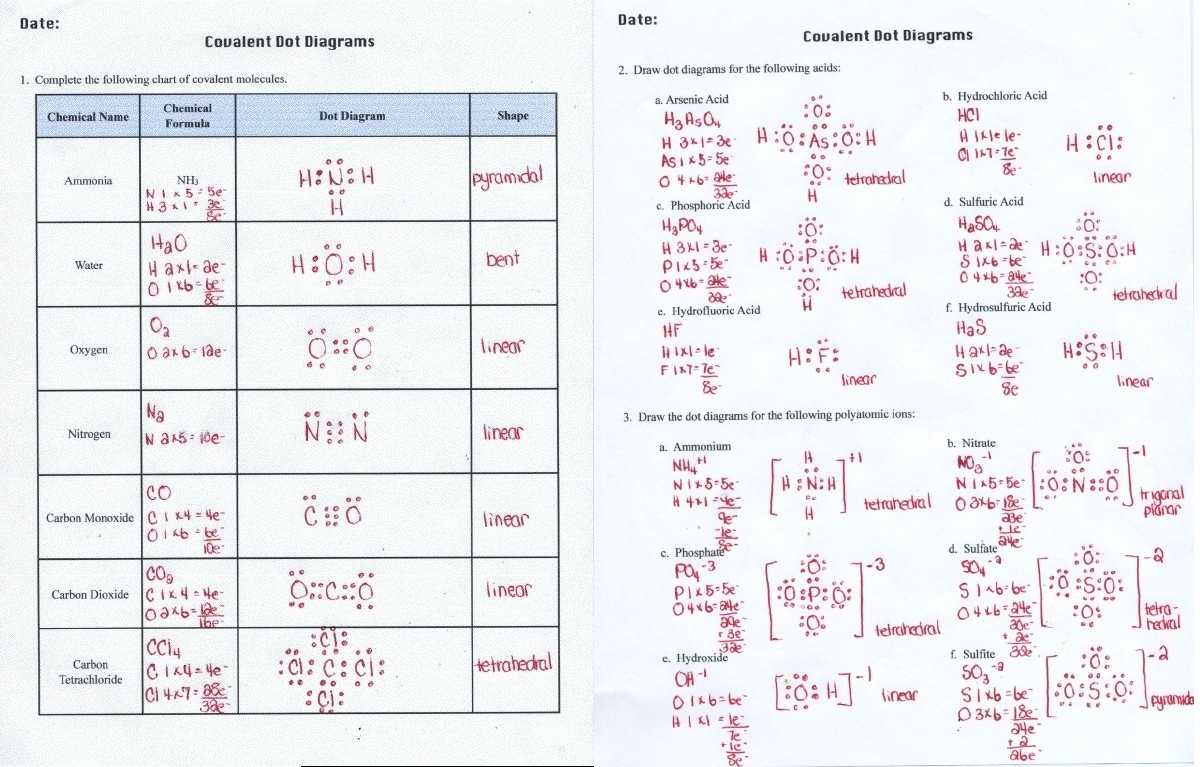
Through careful examination of the Lewis Dot Structure Worksheet Answers, students can learn the electron pair sharing and bond polarity associated with the molecule. This information can be used to determine the type of bonding and to identify the intermolecular forces present in the system. Additionally, students can use the worksheet to predict the shape of the molecule and to identify the number and type of lone pairs of electrons.
By utilizing the Lewis Dot Structure Worksheet Answers, students can gain an understanding of the molecular structure and the type of bonding present in the molecule. This knowledge can be applied to their studies of the properties of molecules, including the reactivity and polarity of the molecule. Additionally, students can use the worksheet to calculate the energy of a reaction, giving them a better understanding of the thermodynamics of the system.
Overall, Lewis Dot Structure Worksheet Answers can be a powerful tool for improving a student’s chemistry grade. With the help of this worksheet, students can gain an understanding of the molecular structure and the type of bonding present in the molecule, which can be applied to their studies of the properties of molecules. Additionally, students can use the worksheet to calculate the energy of a reaction, giving them a better understanding of the thermodynamics of the system. With diligent use of the worksheet, students can improve their knowledge of chemistry and improve their grades.
Conclusion
In conclusion, Lewis Dot Structure Worksheet Answers can be a great resource for students to learn about the structure of molecules and their bonding. It can also serve as a useful tool for students to practice their understanding of chemical bonds and the properties of different types of molecules. With the help of this worksheet, students can learn to create accurate Lewis Dot Structures and understand the importance of their structure in different processes and reactions.
[addtoany]