Exploring the Benefits of Using an Ionic Bonds Worksheet Answer Key
An ionic bond worksheet answer key is an invaluable tool for students of chemistry. It provides them with the answers to the questions they have been assigned so that they can understand the concepts of ionic bonds better. It also helps them understand how the different elements interact with each other in order to form the bonds, and how the bond strength is affected by the charges of the elements involved.
Using an ionic bonds worksheet answer key can help students to better understand the concepts of ionic bonds and the process of forming them. It can also be used to practice the concepts and to test their understanding of these concepts by answering a variety of questions and checking the answers against the worksheet answer key. This can help them to become more proficient and confident in their understanding of the topics.
The worksheet answer key can also be used to reinforce the concepts and to help students to remember the concepts better. By providing students with the answers to the questions, they can easily refer back to the answer key to ensure that they are still on the right track. This can be particularly useful when they are studying for exams or writing essays on the subject.
[toc]
Using an ionic bonds worksheet answer key can help to improve student performance in chemistry. Students can use the worksheet answer key to identify areas in which they need to focus more on in order to understand the concepts better. They can also use the worksheet answer key to identify any errors or inconsistencies in their understanding of the concepts. This can help them to improve their performance and to become more knowledgeable in the subject.
Overall, an ionic bonds worksheet answer key can be a great tool for students of chemistry. It can help them to better understand the concepts and to practice the concepts more effectively. It can also help them to identify any errors or inconsistencies in their understanding of the concepts, which can help them to become more proficient in the subject.
How to Read and Understand an Ionic Bonds Worksheet Answer Key
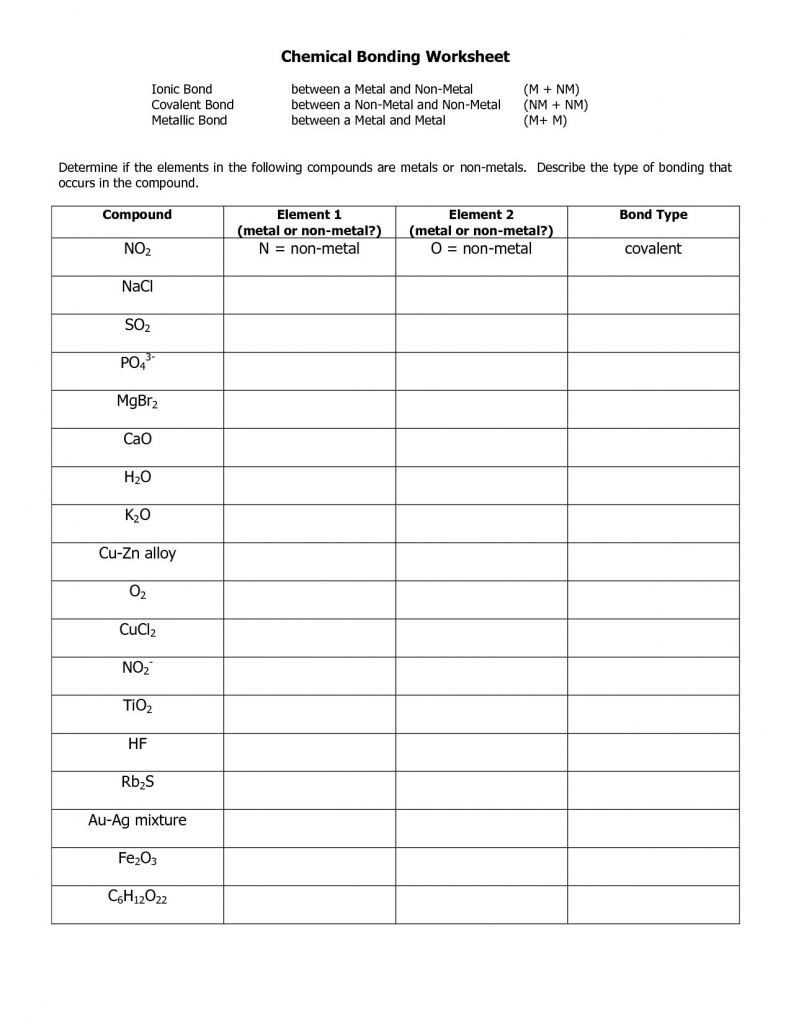
1. Understand the basics of an ionic bond. An ionic bond is an electrostatic force of attraction between two oppositely charged ions. It is formed when one atom transfers electrons to another atom.
2. Identify the type of elements involved. An ionic bond is typically formed between a metal and a nonmetal. The metal atom will lose electrons and become a positively charged ion while the nonmetal atom will gain electrons and become a negatively charged ion.
3. Look for the symbols used to represent the elements. The symbols used to represent the elements involved in an ionic bond are typically listed on the worksheet answer key. The symbols are usually the elemental symbols of the atoms involved in the bond.
4. Examine the charges of the elements. The worksheet answer key will typically list the charges of the elements involved in the ionic bond. The metal atom will have a positive charge while the nonmetal atom will have a negative charge.
5. Determine the number of electrons gained or lost. The worksheet answer key should also list the number of electrons gained or lost by the atoms involved in the bond. The metal atom will lose electrons while the nonmetal atom will gain electrons.
6. Create the chemical formula. The worksheet answer key should include the chemical formula for the ionic bond. The formula will typically include the symbols for the elements involved in the bond along with their respective charges. Additionally, the number of electrons gained or lost by each atom should be included.
Utilizing Ionic Bonds Worksheet Answers to Reinforce Learning
Ionic bonds are a type of chemical bond that form when two ions with opposite charges come together. Ionic bonds are especially important in the formation of salts, which are the compounds that are produced when an acid and a base react with each other. To reinforce learning about ionic bonds, the following worksheet can be used.
1. What is an ionic bond?
An ionic bond is a type of chemical bond that forms when two ions with opposite charges come together. The positively charged ion, or cation, is attracted to the negatively charged ion, or anion, and it is this attraction that forms the strong bond between the two ions.
2. What are the characteristics of an ionic bond?
Ionic bonds are strong bonds that are typically formed between atoms with a large difference in electronegativity. Because of this difference, the atom that is more electronegative tends to draw electrons away from the other atom, forming a cation and an anion. These ions are then attracted to each other, forming an ionic bond.
3. What types of atoms form ionic bonds?
Ionic bonds are typically formed between a metal and a nonmetal. The metal atom tends to lose one or more electrons to the nonmetal atom, forming a cation and an anion, which are then attracted to each other, forming an ionic bond.
4. What are examples of ionic bonds?
Common examples of ionic bonds include the bond between sodium and chlorine, which forms sodium chloride (NaCl), and the bond between calcium and oxygen, which forms calcium oxide (CaO). Other examples include the bond between magnesium and oxygen, which forms magnesium oxide (MgO), and the bond between potassium and sulfur, which forms potassium sulfide (K2S).
The Basics of Creating an Ionic Bonds Worksheet Answer Key
1. What is an ionic bond?
An ionic bond is a type of chemical bond that is formed when one or more electrons from one atom are transferred to another atom. This results in the formation of ions with opposite charges that are attracted to each other and form a strong bond.
2. What is the difference between covalent and ionic bonds?
Covalent bonds involve the sharing of electrons between atoms, whereas ionic bonds involve the complete transfer of electrons from one atom to another. Covalent bonds are generally weaker than ionic bonds, but they are more common in organic molecules.
3. What elements form ionic bonds?
Ionic bonds are typically found between atoms of elements in the same group on the periodic table. Elements such as sodium, potassium, calcium, and magnesium commonly form ionic bonds.
4. What is a Lewis structure?
A Lewis structure is a diagram that represents the arrangement of atoms and electrons in a molecule. Each atom is represented by its symbol and is surrounded by dots that represent valence electrons. A Lewis structure is used to analyze the structure and reactivity of a molecule.
5. What is an example of an ionic bond?
An example of an ionic bond is the bond between sodium and chlorine. Sodium has one electron in its outermost shell and chlorine has seven, so when they react, sodium donates its electron to chlorine and they form an ionic bond.
Conclusion
In conclusion, the Ionic Bonds Worksheet Answers provide a great resource for students to learn the basics of ionic bonds and their properties. It is a great way to understand the formation and structure of ionic bonds and how they interact with other molecules. The worksheet also provides an opportunity to practice the concepts discussed in class. With the help of this worksheet, students can easily master the concepts of ionic bonds and its applications.
[addtoany]