Exploring the Development of Atomic Theory Through the History of the Atom Worksheet
Atomic theory has been an area of scientific exploration for centuries. The history of the atom has been filled with progress and setbacks, as scientists have sought to understand the structure and behavior of atoms. This worksheet will explore the development of atomic theory over time, beginning with ancient Greek philosopher Democritus and ending with modern day quantum mechanics.
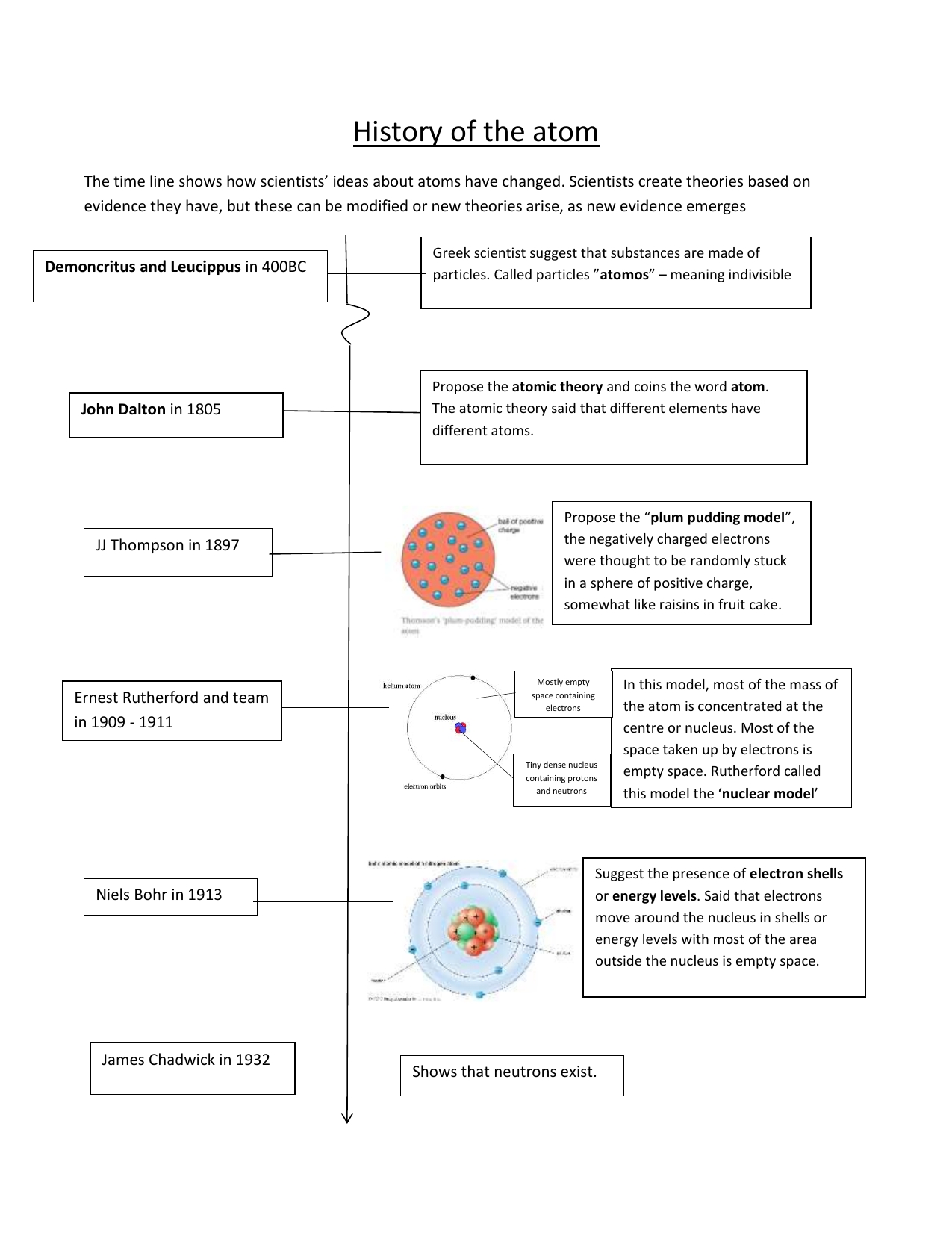
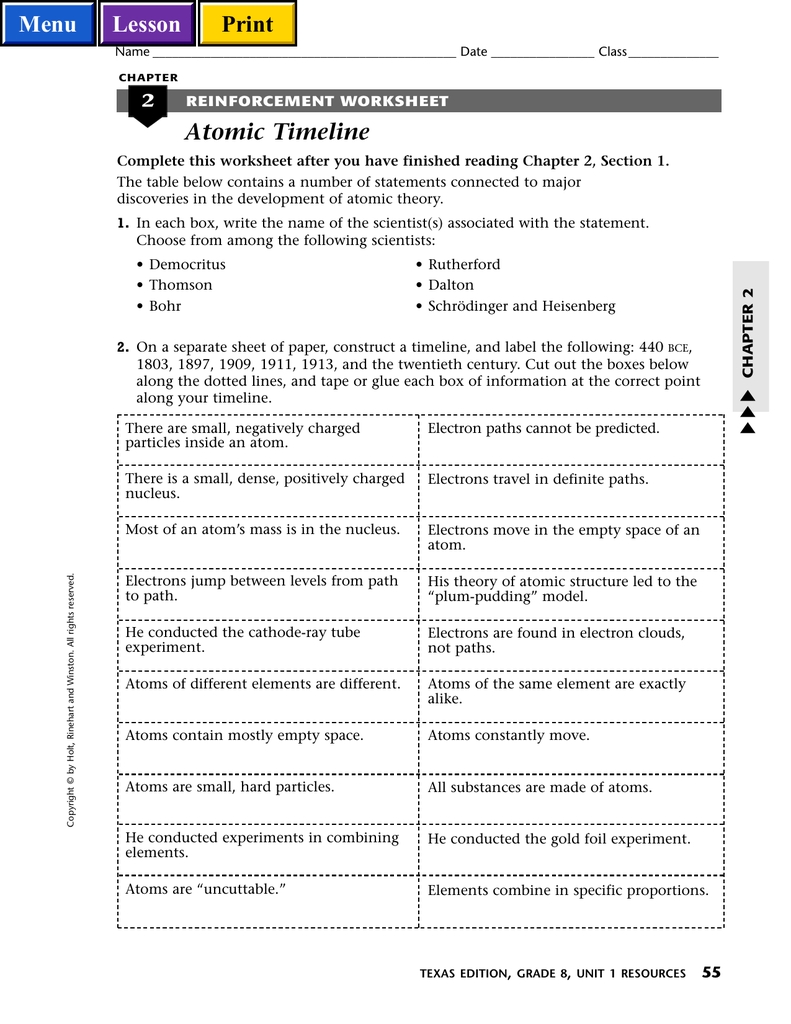
Democritus was the first to propose the existence of atoms. He suggested that all matter is composed of tiny, indivisible particles that are too small to be seen by the human eye. He used the Greek word atomos to describe the idea. This concept was later refined by the Roman philosopher Lucretius, who suggested that atoms could be made up of different shapes, sizes, and weights, and that they could move and interact with each other.
In the 17th century, English chemist Robert Boyle proposed the concept of atoms combining together to form molecules. He argued that different molecules have different properties and that these properties could be explained by the number and arrangement of atoms. Boyle’s work was later expanded upon by French chemist Antoine Lavoisier, who identified the law of conservation of mass. This law states that mass can be neither created nor destroyed, and that any given reaction must have the same amount of mass before and after the reaction.
[toc]
In the 19th century, English chemist John Dalton proposed the idea of an atomic theory of matter. He suggested that all elements are made up of atoms, and that these atoms can combine together to form compounds. He also proposed that atoms of the same elements are identical in weight and size, and that the weight of each atom is constant. Dalton’s work was later expanded upon by Italian physicist Amedeo Avogadro, who suggested that different atoms have different numbers of electrons. This idea led to the concept of atoms having different properties, such as electronegativity and ionization energy.
In the early 20th century, Danish physicist Niels Bohr proposed the Bohr model of the atom. This model suggests that atoms are made up of a nucleus surrounded by electrons that orbit in specific shells. The shells contain different amounts of energy, and the electrons can jump between them. This model provided insight into the structure and behavior of atoms, and was later expanded upon by German physicist Werner Heisenberg and Austrian physicist Erwin Schrödinger.
Today, modern day quantum mechanics explains the behavior of atoms on a subatomic level. This theory suggests that electrons exist in a wave-like form, and that their behavior can be described by mathematical equations. This provides a more detailed understanding of atomic structure and behavior, which is used to explain many phenomena in nature.
The history of the atom has been filled with progress and discoveries, as scientists have sought to understand the structure and behavior of atoms. This worksheet has explored the development of atomic theory over time, beginning with ancient Greek philosopher Democritus and ending with modern day quantum mechanics. By understanding the history of the atom, we can gain greater insight into the nature of matter, and the laws governing its behavior.
The Impact of the Atom Worksheet on Understanding Atomic Structure
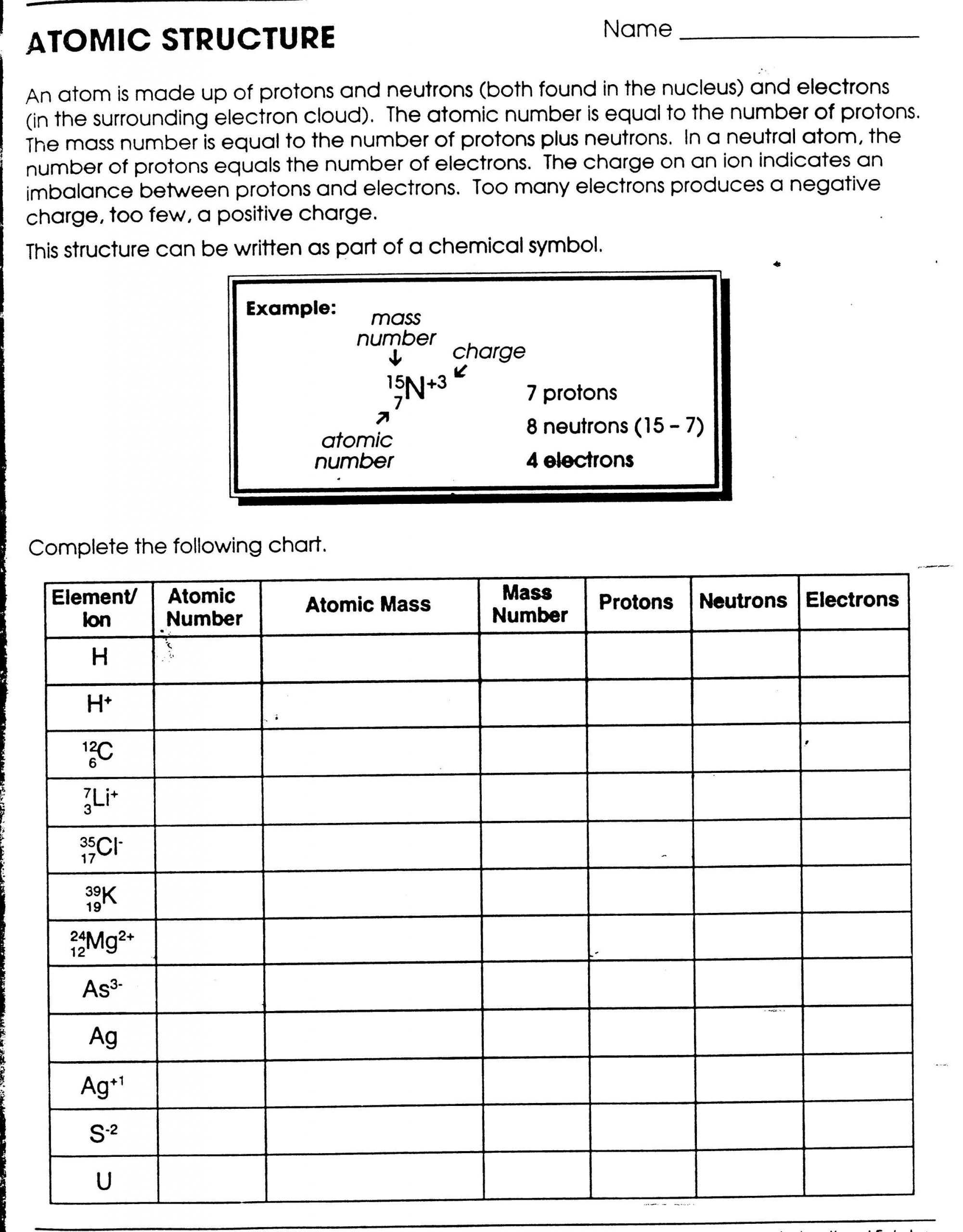
The Atom Worksheet offers a systematic and thorough approach to understanding atomic structure. It provides a comprehensive overview of the fundamental principles of atomic structure, including the structure of the nucleus, the nature of the electron clouds, and how the protons, neutrons and electrons are arranged.
The worksheet is divided into four sections, each focusing on a particular aspect of atomic structure. The first section covers the basics of the structure of the nucleus, including the number of protons and neutrons, their location within the nucleus, and the arrangement of the protons and neutrons in a pattern known as the nuclear shell model. The second section explains the nature of the electron clouds, including their size and shape, and how they interact with other particles. The third section outlines the structure of the atom, including the number of electrons, their arrangement around the nucleus, and how they are arranged in the various shells. Finally, the fourth section summarizes the relationship between the nucleus and the electron clouds, and how this relationship affects the properties of the atom.
Overall, the Atom Worksheet provides a comprehensive and accessible introduction to atomic structure. It clearly outlines the fundamental principles of atomic structure and provides a comprehensive overview of how the protons, neutrons and electrons are arranged in the nucleus and electron clouds. By providing an easy-to-understand explanation of the basic principles, the worksheet provides an invaluable tool for any student seeking to gain a better understanding of atomic structure.
Uncovering the Role of the Atom Worksheet in Shaping Nuclear Physics
Nuclear physics is one of the most important fields of study in the physical sciences. It is a complex and fascinating area of research, with a long and storied history. One tool that has been integral to the development of nuclear physics is the Atom Worksheet. This tool has been used to investigate the structure of the atom and to provide insights into the behavior of nuclear systems.
The Atom Worksheet is a simple yet powerful tool that allows scientists to explore the structure of the atom and its components. The worksheet is composed of a grid of squares and symbols, which represent the nucleus of the atom and the electrons that orbit it. By arranging the symbols on the grid, scientists can gain an understanding of the structure of the nucleus and its components, as well as the behavior of the electrons.
The Atom Worksheet can be used to investigate a variety of different topics within nuclear physics. Scientists can use it to explore the properties and behavior of different types of particles, such as neutrons and protons. They can also use it to understand the structure of nuclei, and to investigate the properties of different isotopes. By examining the structure of the atom and its components, scientists can gain insights into the nature of nuclear interactions, such as fission and fusion.
The Atom Worksheet has also been used to investigate the properties of radioactive materials. By examining the position and size of the atoms on the worksheet, scientists can gain an understanding of the properties of the radioactive material. They can also use the worksheet to understand the interactions between different nuclear materials, such as uranium and plutonium.
The Atom Worksheet has been an important tool in the progress of nuclear physics over the years, providing a valuable source of data and insights. It has been used to investigate a wide range of topics, from the structure of the nucleus to the behavior of radioactive materials. Through its use, scientists have been able to gain a better understanding of the structure of the atom and its components, as well as the behavior of nuclear systems. As such, the Atom Worksheet has played an essential role in shaping the field of nuclear physics.
Conclusion
The History of the Atom Worksheet showed us how our understanding of atom structure has evolved over time. From the ancient Greeks who first proposed the concept of the atom, to modern scientists using technology to map the atom’s structure and behavior, we have come a long way in understanding this fundamental building block of matter. Through this worksheet, we have gained a better understanding of the atom and its role in the universe.
[addtoany]