Exploring Boyle’s Law Worksheet Answers: A Comprehensive Guide
I. Introduction
Robert Boyle is one of the most influential figures in the history of science. His work in the 17th century laid the groundwork for the modern understanding of chemistry and physics. Boyle’s Law is one of the most important discoveries made by Boyle and is still used today. In this worksheet, we will explore Boyle’s Law and its implications for the study of chemistry and physics.
II. What is Boyle’s Law?
[toc]
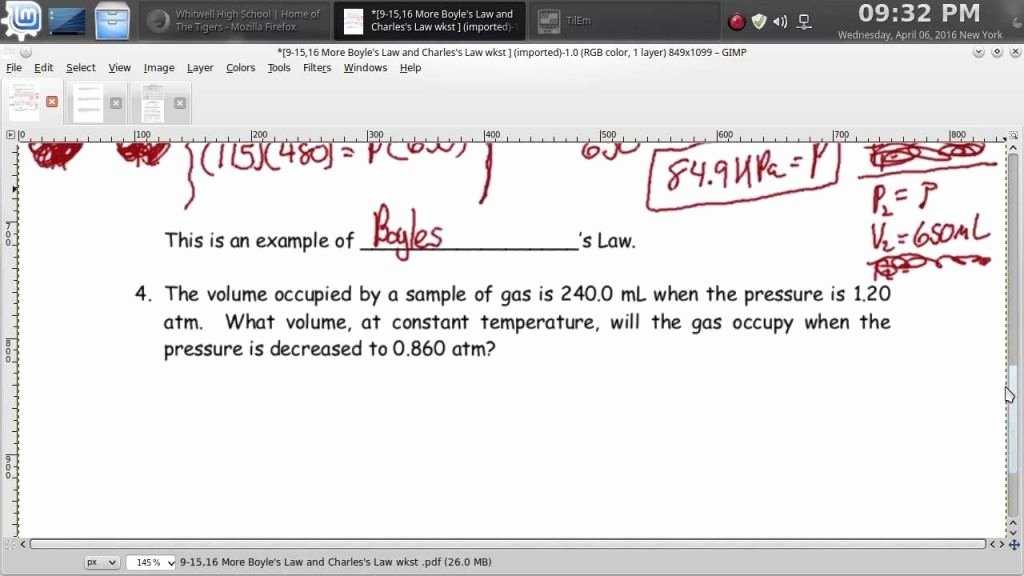
Boyle’s Law states that the pressure of a given amount of gas, held at a constant temperature, is inversely proportional to its volume. In other words, when the volume of a gas is halved, its pressure will double, and when the volume of a gas is doubled, its pressure will be halved. This relationship is expressed mathematically as PV = k, where P is the pressure, V is the volume and k is a constant.
III. Applications of Boyle’s Law
Boyle’s Law can be used to calculate the pressure of a gas given its volume and temperature. It can also be used to calculate the volume of a gas given its pressure and temperature. Finally, it can be used to calculate the pressure of a gas in a sealed container, such as an aerosol can or a balloon.
IV. Implications of Boyle’s Law
Boyle’s Law has important implications for the study of gases and the behavior of gases under different conditions. It can be used to explain the behavior of gases in a closed container, such as a balloon, and to predict the pressure of a gas given its volume and temperature. It also explains the phenomenon of compression and expansion, which is important for understanding the behavior of gases in engines and other mechanical systems.
V. Conclusion
Boyle’s Law is an important and influential discovery made by Robert Boyle in the 17th century. It states that the pressure of a given amount of gas, held at a constant temperature, is inversely proportional to its volume. This relationship has important implications for the study of gases and the behavior of gases under different conditions. Its applications range from calculating the pressure of a gas given its volume and temperature to predicting the pressure of a gas in a sealed container. Boyle’s Law remains a cornerstone of modern chemistry and physics.
Analyzing Boyle’s Law Worksheet Answers: What Can We Learn?
Boyle’s Law is a fundamental scientific concept that explains the behavior of gases in a closed system. By studying Boyle’s Law worksheets, students can learn how to apply the law to various scenarios and gain a deep understanding of its implications.
The worksheets generally consist of multiple-choice questions, problems, and diagrams that require students to calculate the relationships between pressure, volume, and temperature of gases. Such questions can give students an understanding of the inverse nature of Boyle’s Law, which states that the pressure and volume of a gas are inversely proportional. This means that if the pressure of a gas increases, its volume decreases, and vice versa.
By working through the problems on Boyle’s Law worksheets, students can also gain insights into the effects of temperature on the behavior of gases. Temperature can affect the pressure and volume of a gas, and the worksheets can help students to understand the implications of this.
Finally, the worksheets can also provide students with an opportunity to apply what they have learned in a practical context. By completing the problems, students can gain a better understanding of how Boyle’s Law can be used to solve real-world problems related to gas behavior.
Overall, Boyle’s Law worksheets can provide students with an invaluable opportunity to gain a deeper understanding of the principles of gas behavior. Through the questions and problems, students can learn how to calculate the relationships between pressure, volume, and temperature of gases, as well as understand the implications of temperature and its effects on the behavior of gases. By completing the worksheets, students can also gain practical experience in solving real-world problems related to gas behavior.
Unpacking Boyle’s Law Worksheet Answers: Breaking Down Complex Concepts
Boyle’s Law states that the volume of a gas is inversely proportional to the pressure of the gas. This law worksheet explores the different aspects of this law, providing students with an opportunity to understand the concepts and apply them to real-world situations.
The worksheet begins by introducing Boyle’s Law and its significance. It then moves on to explaining the formula for Boyle’s Law and its implications. Students are asked to calculate the pressure of a gas given its volume, and vice versa. They are also asked to use the formula to solve for pressure and volume in various scenarios.
The worksheet then dives deeper into the topic by exploring how Boyle’s Law applies to the real world. Students are asked to calculate the pressure of a gas in a container when the temperature is changed. They are also asked to relate the pressure of a gas to the atmospheric pressure, and to use Boyle’s Law to calculate the gas pressure at different altitudes.
Finally, the worksheet provides students with a few challenging questions on Boyle’s Law. Students are asked to solve a multi-step problem, and to determine how the pressure of a gas changes in certain conditions.
The worksheet is designed to help students gain a better understanding of Boyle’s Law, and to apply it to different scenarios. By breaking down complex concepts into easy-to-understand formulas and examples, students will be able to gain a thorough understanding of this law and how it applies to the real world.
Conclusion
Boyle’s Law Worksheet Answers provides a comprehensive overview of the important concepts related to Boyle’s Law. It is a great resource for anyone wanting to learn more about this scientific law and how it applies to various real-world scenarios. With its easy-to-follow explanations of the concepts and its worked examples, this worksheet is an invaluable tool for helping students understand the principles of Boyle’s Law.
[addtoany]