How to Interpret the Results of a Bohr Atomic Models Worksheet Answer
Interpreting the results of a Bohr atomic model worksheet is a process that requires careful consideration. A Bohr atomic model worksheet typically consists of a set of questions related to the properties of an atom and the various components of its structure. In order to properly interpret the results, one must be familiar with the principles behind the Bohr atomic model, which includes the nuclear model, orbitals, and the atomic number.
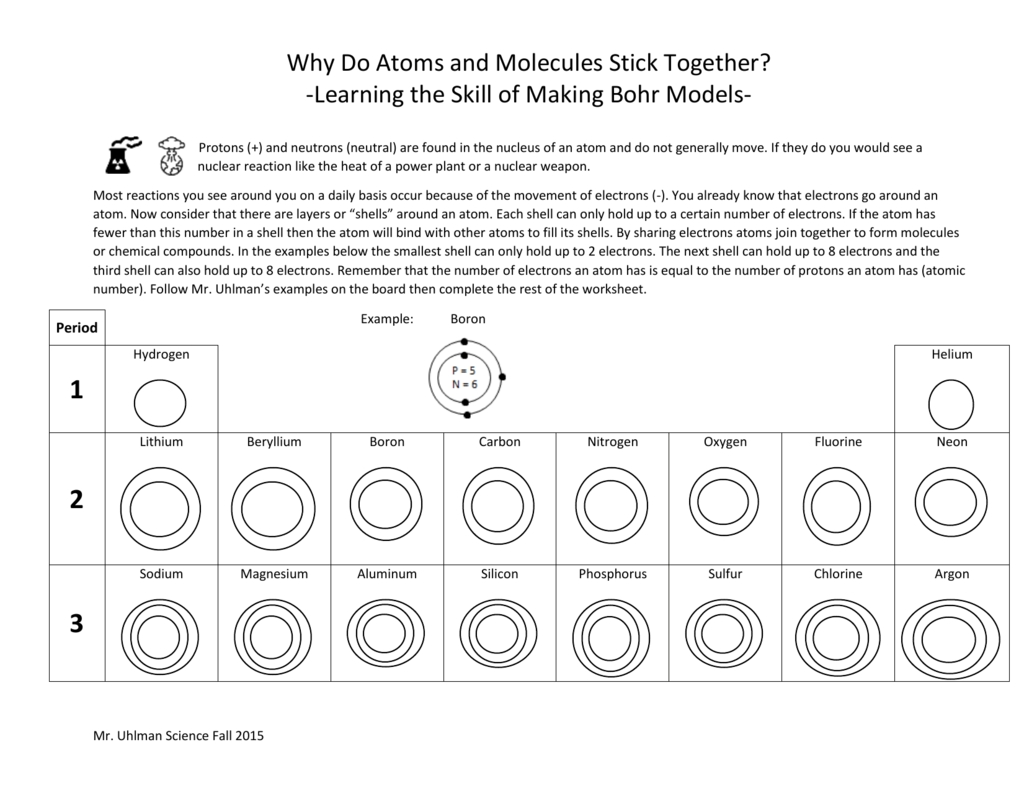
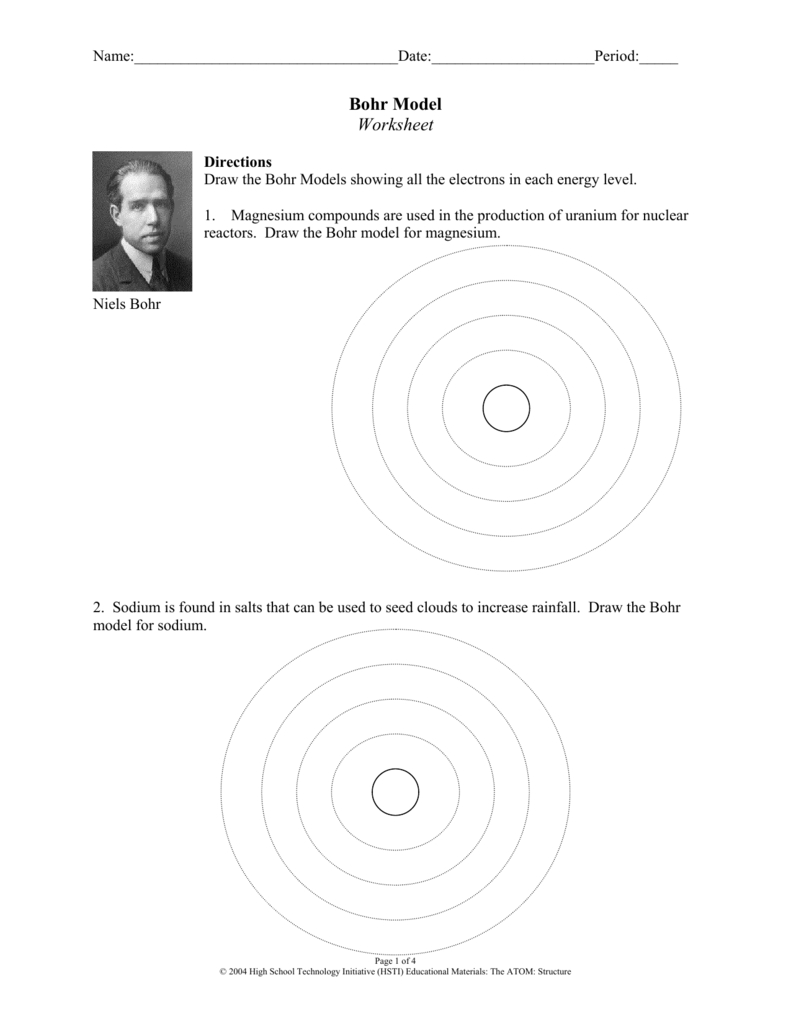
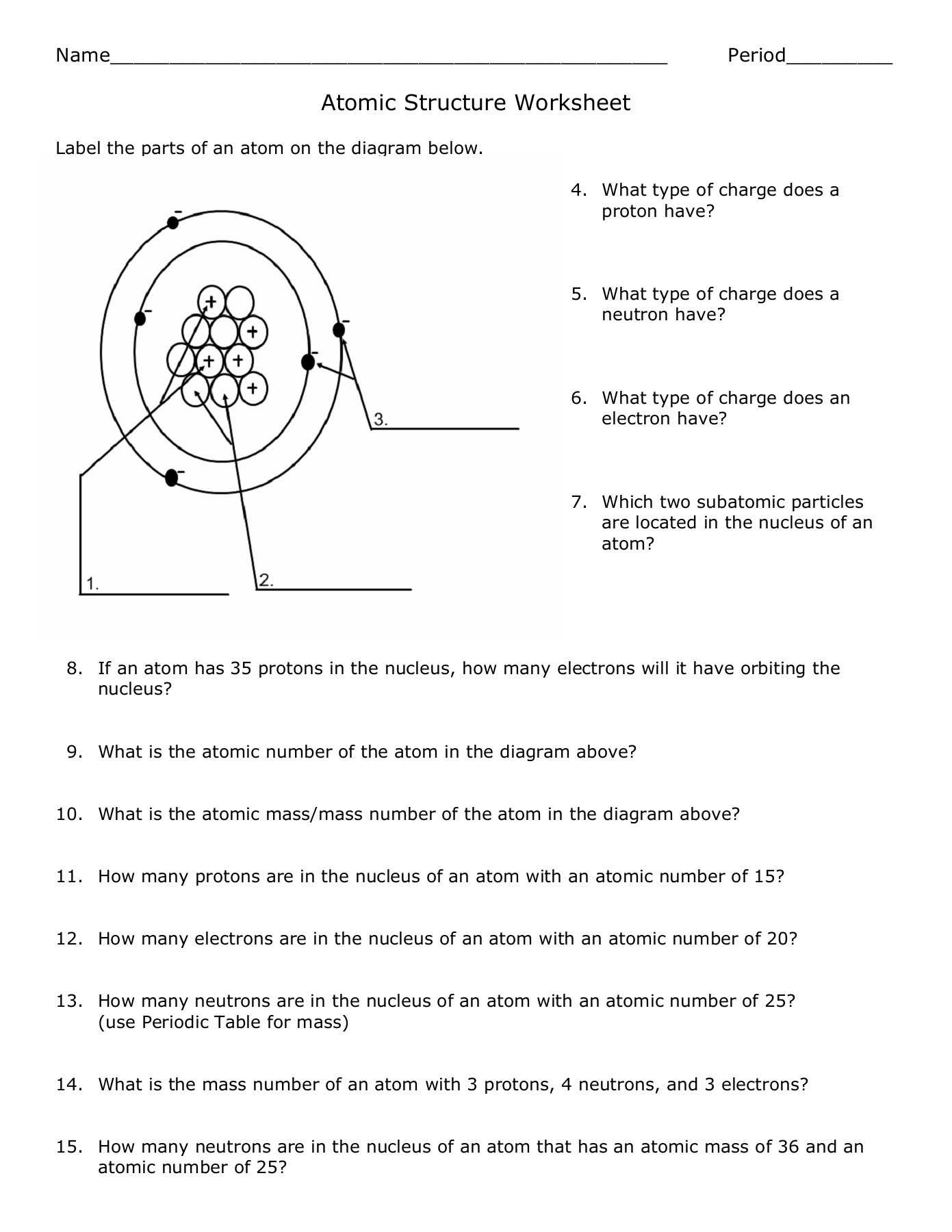
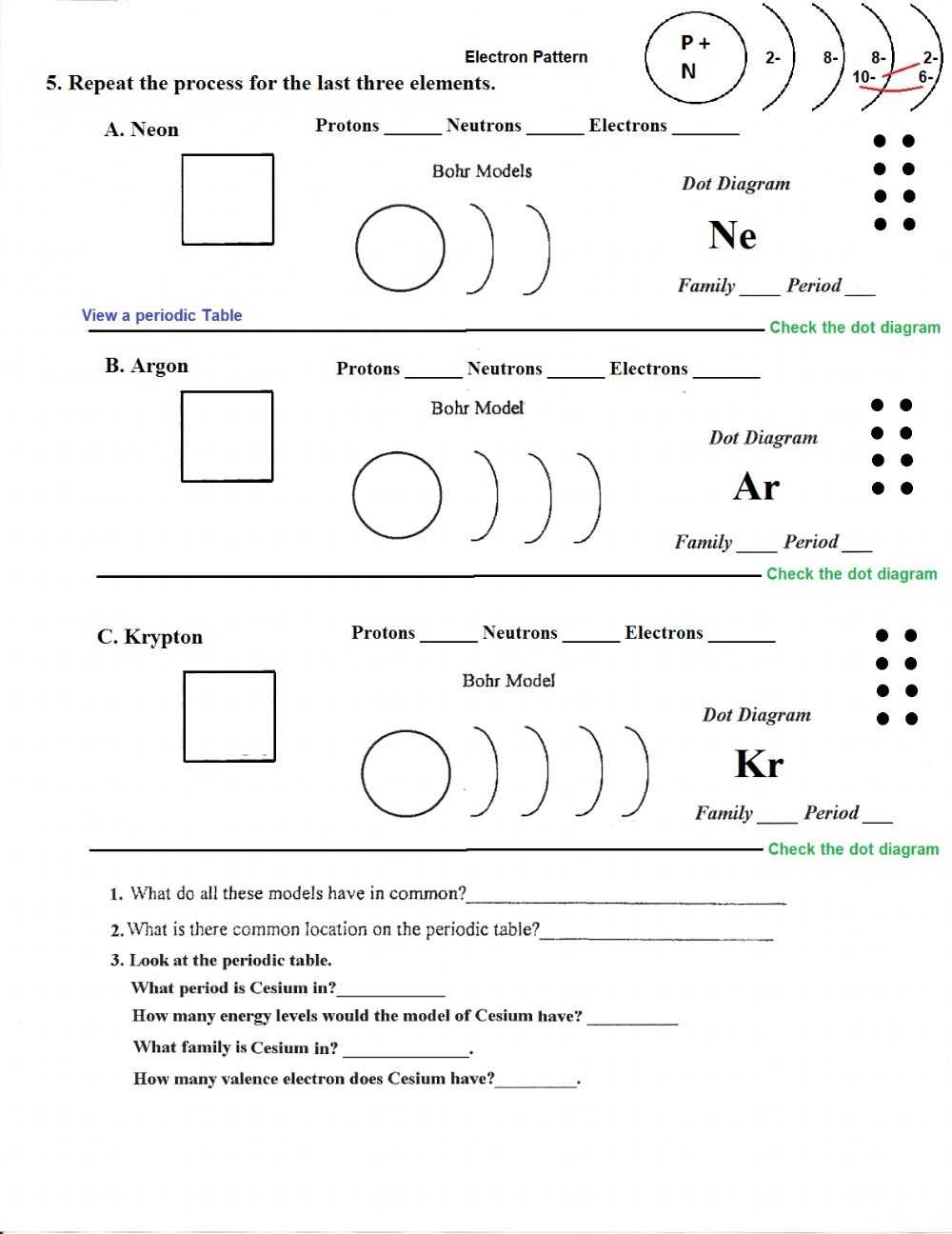
The first step in interpreting the results of a Bohr atomic model worksheet is to identify the atom that is being modeled. This can be done by examining the atomic number or the elements on the periodic table. Once the atom has been identified, the next step is to analyze the questions on the worksheet and determine which parts of the Bohr atomic model are being addressed. For example, questions may ask about the number of protons, neutrons, and electrons in the atom, as well as the orbitals and the energy levels associated with them.
After all of the questions have been answered, the next step is to analyze the data and draw conclusions. The answers to the questions can be used to determine the properties of the atom, such as its mass and size. Additionally, the orbitals can be used to determine the energy levels of the atom and how these interact with each other.
[toc]
Finally, the results of the Bohr atomic model worksheet can be compared to existing models or used to create new models. By doing so, one can gain a better understanding of how atoms interact and how they behave in different situations. By interpreting the results of the Bohr atomic model worksheet, one can gain a more comprehensive understanding of the structure and behavior of atoms.
Exploring the Relationship Between Bohr Atomic Models and Chemistry
The Bohr atomic model is an integral part of the understanding of chemistry, providing a comprehensive and accurate view of the behavior of atomic particles. Developed in the early 20th century, the model is based on the idea of a stationary atom, with a nucleus at its center, composed of protons and neutrons, surrounded by orbiting electrons. This model offers an explanation of the behavior of atoms and their reactions, as well as the chemical properties of various elements.
In the Bohr model, the nucleus of the atom is surrounded by a series of concentric shells, which contain the electrons. The shells are organized according to the energy of the electrons and their distance from the nucleus, as determined by the quantum numbers associated with them. Each shell can hold a certain number of electrons, and the electrons can move between shells as they gain and lose energy. This energy is related to the attraction of the nucleus and the electrons, as well as the repulsion between the electrons, and it helps to determine the chemical properties of the atom.
The Bohr model offers an explanation of the behavior of the electrons and their interactions with other atoms. It explains why atoms bond and how they form chemical compounds. It also explains why atoms form molecules with specific shapes and sizes, and how they react with other atoms. By understanding the behavior of the electrons, chemists can better understand the chemical properties of various elements.
The Bohr model has also been used to explain the behavior of light, which is composed of photons, or particles of energy. By understanding the behavior of the photons, scientists can better understand the emission and absorption of light, which has implications for the study of chemical reactions.
In conclusion, the Bohr atomic model is essential to the understanding of chemistry. By understanding the behavior of electrons and photons, chemists can gain a better understanding of the chemical properties of various elements and how they interact. This understanding can then be used to inform experiments and research, leading to further advances in the field.
Using Bohr Atomic Models Worksheet Answers to Make Predictions about Atomic Structure
Using Bohr atomic models, scientists have been able to make predictions about atomic structure. Bohr’s atomic model is a representation of the atom that explains its behavior and structure. It postulates that the electrons in an atom move around the nucleus in circular orbits and that each orbit is a distinct energy level. This model explains the behavior of electrons in an atom and predicts the energy levels at which electrons can exist.
By examining the structure of the atom, scientists can make predictions about the size of the atom and the number of electrons in each orbit. The number of electrons in each orbit is determined by the number of protons in the nucleus. According to the model, the nucleus has a positive charge, which attracts negatively charged electrons. Thus, the number of protons determines the number of electrons in each orbit.
The size of the atom is determined by the magnitude of the electron orbits. The larger the orbit, the larger the atom. The size of the orbits is determined by the energy level of the electrons. According to the model, electrons in lower energy levels have smaller orbits, while electrons in higher energy levels have larger orbits.
By using Bohr’s atomic model, scientists can make predictions about atomic structure. The number of electrons in each orbit is based on the number of protons in the nucleus and the size of the atom is determined by the magnitude of the electron orbits. This model is a useful tool for understanding the behavior and structure of atoms.
Conclusion
Overall, the Bohr Atomic Models Worksheet Answers provide a comprehensive and informative overview of the Bohr atomic model. By working through this worksheet, students can gain a better understanding of the structure and principles of the Bohr atomic model, as well as gain a better appreciation of the immense complexity of the atom. This worksheet can be used as a helpful tool in helping students to understand the basics of atomic structure and how it relates to the world around us.
[addtoany]