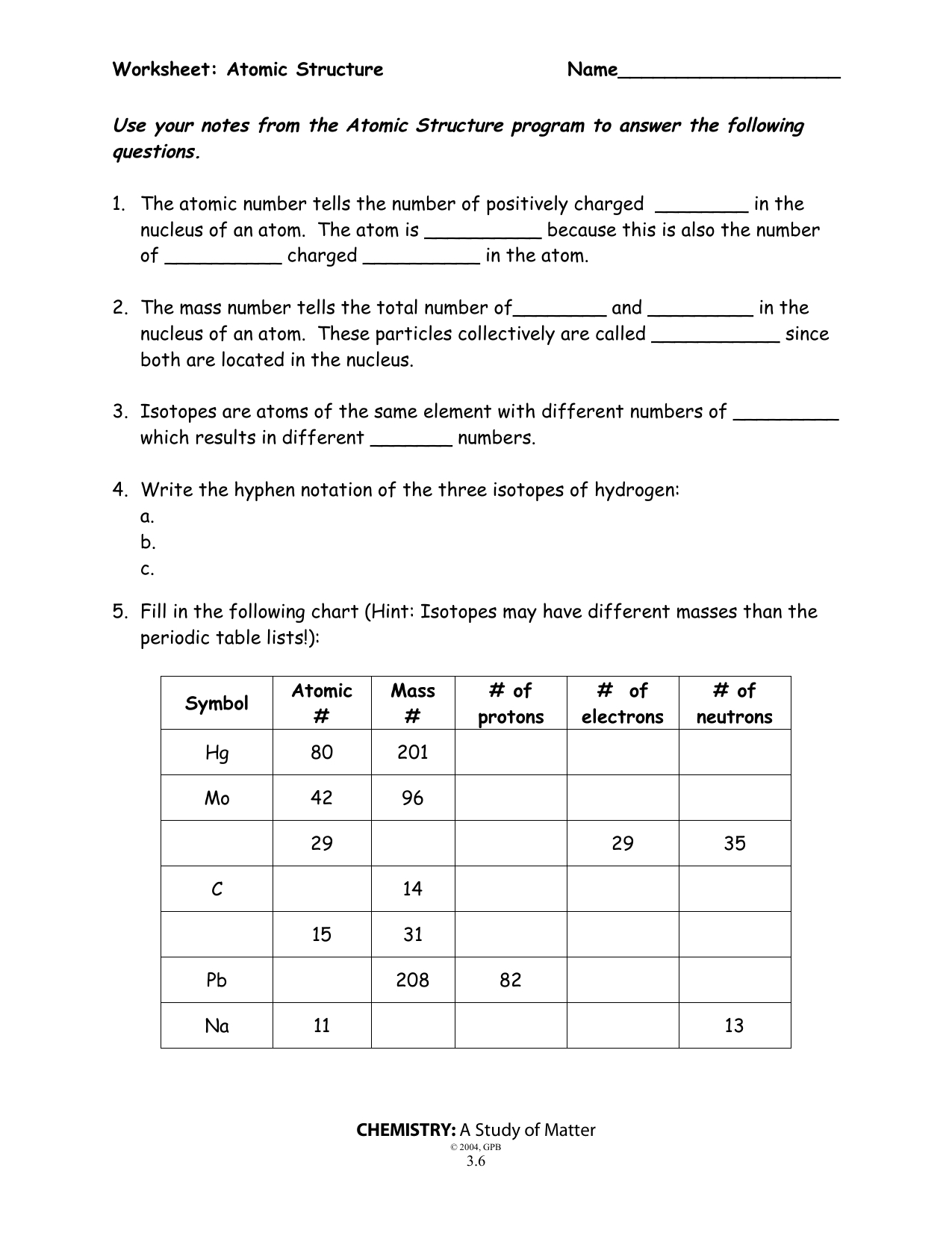
How to Utilize a Basic Atomic Structure Worksheet to Teach Students About Atoms
A basic atomic structure worksheet is a valuable teaching tool for introducing students to the fundamental concepts of atomic structure. It provides an organized and easy-to-follow approach for teaching students about the components of atoms, as well as how they interact with one another. It is an invaluable tool for helping students to gain a deeper understanding of the atomic structure and the associated physics.
When utilizing a basic atomic structure worksheet, it is important to take a step-by-step approach to ensure that students fully understand the material. Begin by introducing the basic concepts of atomic structure, such as the protons, neutrons, and electrons. Explain how these particles interact with one another to form atoms. Emphasize the importance of each component and how it influences the properties of an atom.
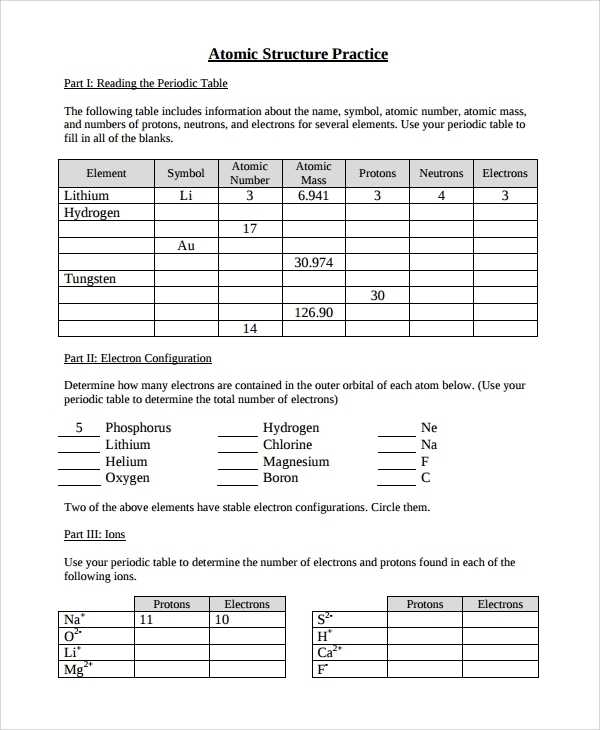
Next, introduce students to the periodic table of elements. Explain how elements are arranged according to their atomic number, mass number, and atomic mass. Demonstrate how to read the periodic table and the different elements that can be found within it. Discuss how different elements interact with one another, and how they form molecules.
[toc]
As students become more familiar with the concepts of atomic structure, introduce them to the Bohr model of the atom. Explain how electrons are arranged around the nucleus in different energy levels. Demonstrate how electrons can absorb and release energy. Discuss how this knowledge can be used to explain the behavior of atoms.
Finally, have students use the worksheet to answer questions regarding the structure and behavior of atoms. Have them practice drawing atoms, labeling the different components, and describing the interactions between the various particles. This will help them to build a solid understanding of the fundamental concepts of atomic structure.
By using a basic atomic structure worksheet, students will be able to gain a better understanding of the fundamentals of atomic structure. It also provides an organized and easy-to-follow approach for teaching students about the components of atoms and how they interact with one another. This is an invaluable tool for helping students to gain a deeper understanding of the atomic structure and the associated physics.
Exploring the Parts of an Atom with a Basic Atomic Structure Worksheet
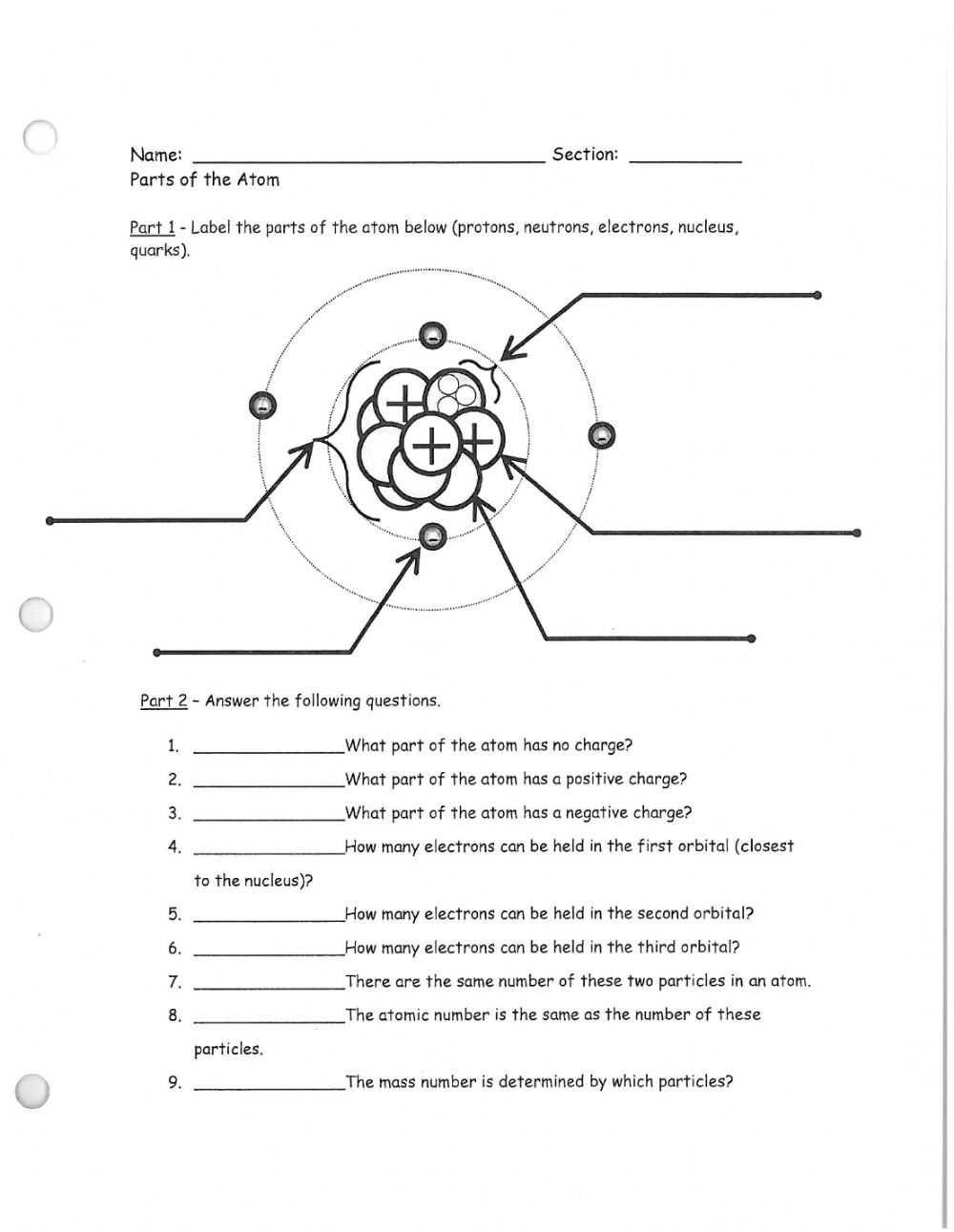
Atoms are the building blocks of the universe, and all matter is composed of them. In this worksheet, we will be exploring the parts of an atom and how they come together to create an atom’s structure.
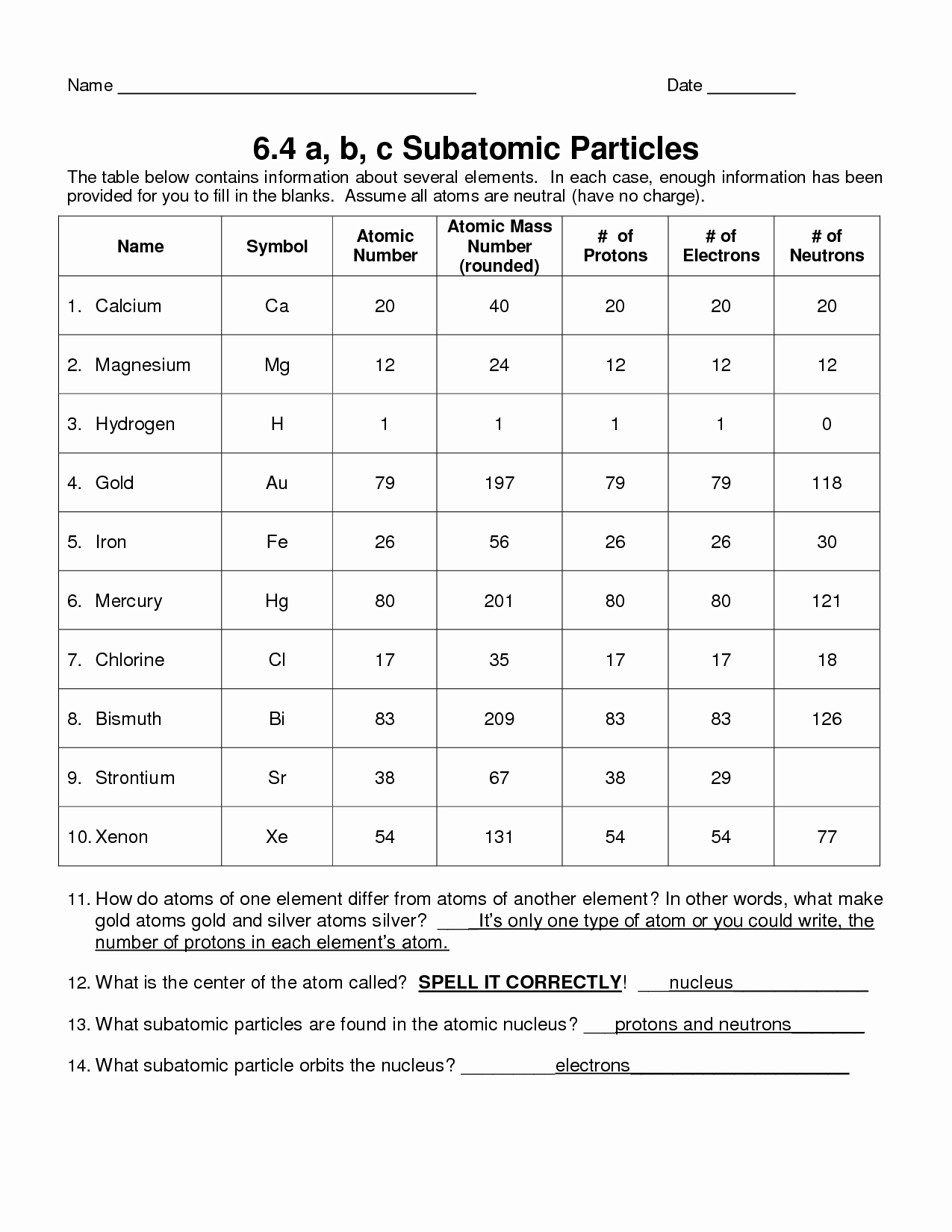
The nucleus is the center of an atom, and it contains most of the atom’s mass. It is composed of protons and neutrons, which are both positively charged particles. Protons have a positive charge of +1, and neutrons have no charge. The number of protons in the nucleus is called the atomic number and it determines an element’s identity.
Surrounding the nucleus is a cloud of electrons. Electrons have a negative charge of -1 and are much smaller than protons and neutrons. Electrons are responsible for most of the chemical properties of an element. The number of electrons in an atom is equal to the number of protons, so the atoms are electrically neutral.
The space between the nucleus and the electrons is called the electron cloud. The electron cloud is a region of uncertainty, and it is impossible to determine the exact position of any electrons inside it.
At the outermost layer of the atom, there are sometimes additional electrons that are not part of the electron cloud. These electrons are referred to as valence electrons, and they are responsible for the chemical bonding between atoms.
An atom’s overall structure can be represented by a diagram called an atomic diagram. An atomic diagram shows the nucleus, the electron cloud, and any additional valence electrons. It also displays the number of protons, neutrons, and electrons in the atom.
By studying this basic structure of an atom, we can gain insight into how atoms interact with each other and form the building blocks of our universe.
Understanding the Properties of Atoms Using a Basic Atomic Structure Worksheet
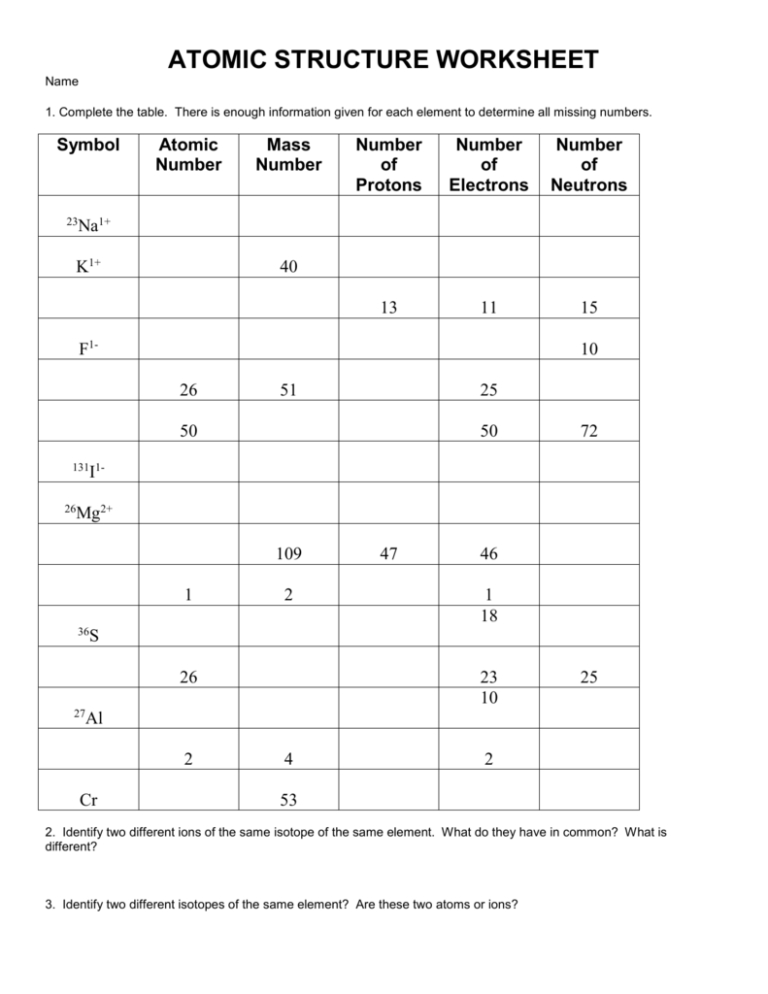
Atoms are the basic building blocks of all matter. A fundamental understanding of the structure of atoms is essential to comprehending the properties of different elements. A basic atomic structure worksheet provides an easy-to-follow visual representation of the parts of an atom, including the nucleus, protons, neutrons, and electrons.
The nucleus is the central core of the atom. It is composed of protons and neutrons. Protons have a positive charge, while neutrons have no charge. Together, they form a strong bond with the electrons, which have a negative charge. The number of protons in an atom is its atomic number, which determines its identity and chemical properties.
Surrounding the nucleus are the electrons, which are the lightest of the particles. They occupy different energy levels, and the number of electrons in an atom is equal to its atomic number. Electrons are responsible for the chemical reactions that occur when atoms interact with each other.
By filling in a basic atomic structure worksheet, students can gain a better understanding of the properties of atoms. The worksheet helps to identify the atomic number and number of electrons of each element. It also explains the differences between protons and neutrons, and how they interact with the electrons. With this knowledge in hand, students can better comprehend the chemical and physical properties of different elements.
Helping Students Learn to Calculate Atomic Mass with a Basic Atomic Structure Worksheet
Calculating atomic mass is an important concept for students in chemistry. This basic atomic structure worksheet is designed to help students better understand the concept of atomic mass.
The worksheet begins by introducing the basic concepts of atomic structure and how the atomic mass is calculated. It also provides a diagram of an atom to help students visualize the different components. Students are then prompted to calculate the atomic mass of a given element using the information provided.
In order to help students better understand the concept, the worksheet also includes a few examples. These examples provide students with a step-by-step explanation of how to calculate the atomic mass. Additionally, the worksheet includes an answer key so that students can check their work.
This worksheet is designed to guide students through the process of calculating atomic mass. By providing students with the basic concepts of atomic structure, examples, and an answer key, this worksheet serves as a great starting point for students to gain a better understanding of atomic mass.
Conclusion
In conclusion, the Basic Atomic Structure Worksheet is a great way to teach students about the basics of atomic structure and its components. It covers a wide range of topics, including the structure of atoms, the different elements that make up atoms, and the different types of bonds between atoms. Students can use the worksheet to gain a deeper understanding of the structure of atoms and the way they interact with each other. This knowledge can be used in future studies of the physical world, such as chemistry and physics.
[addtoany]