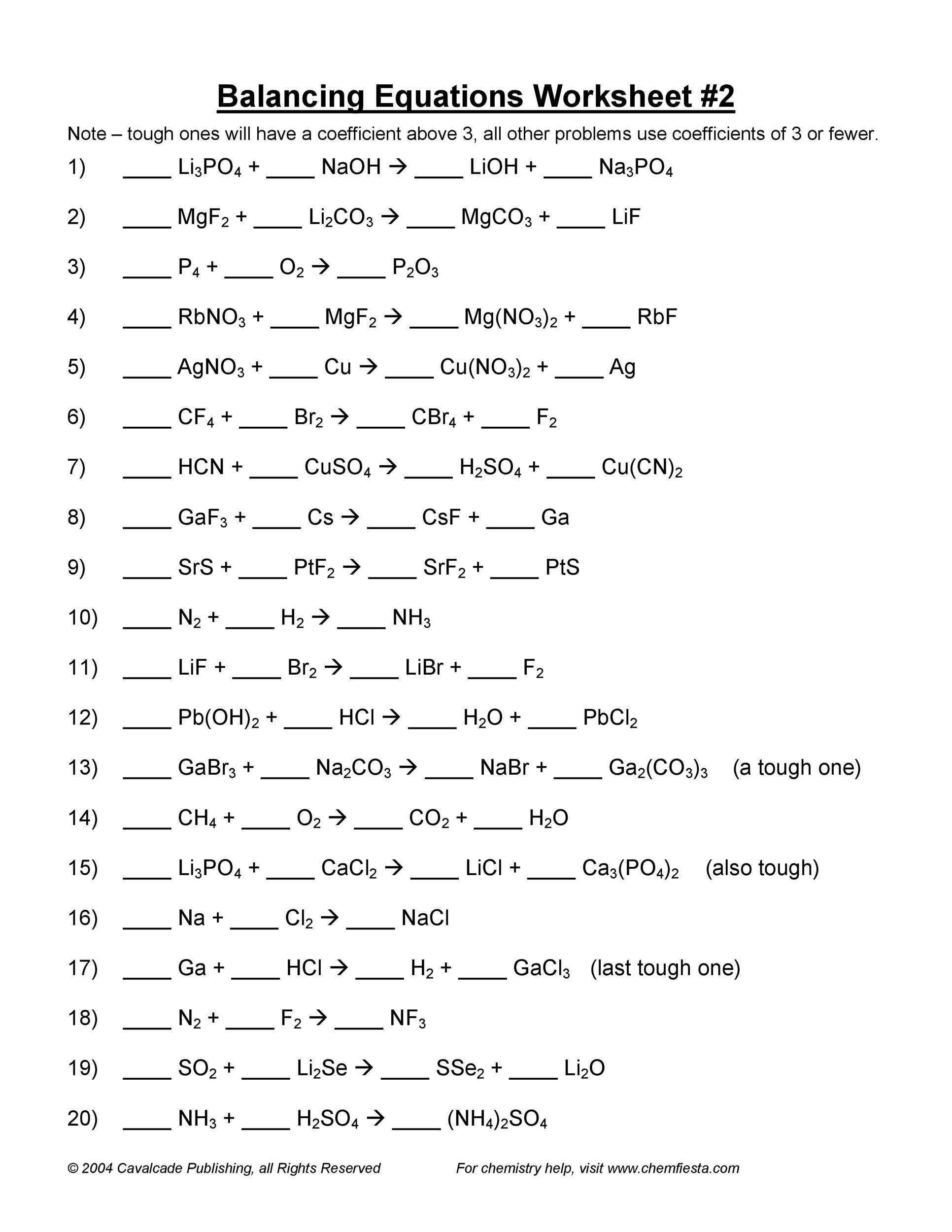
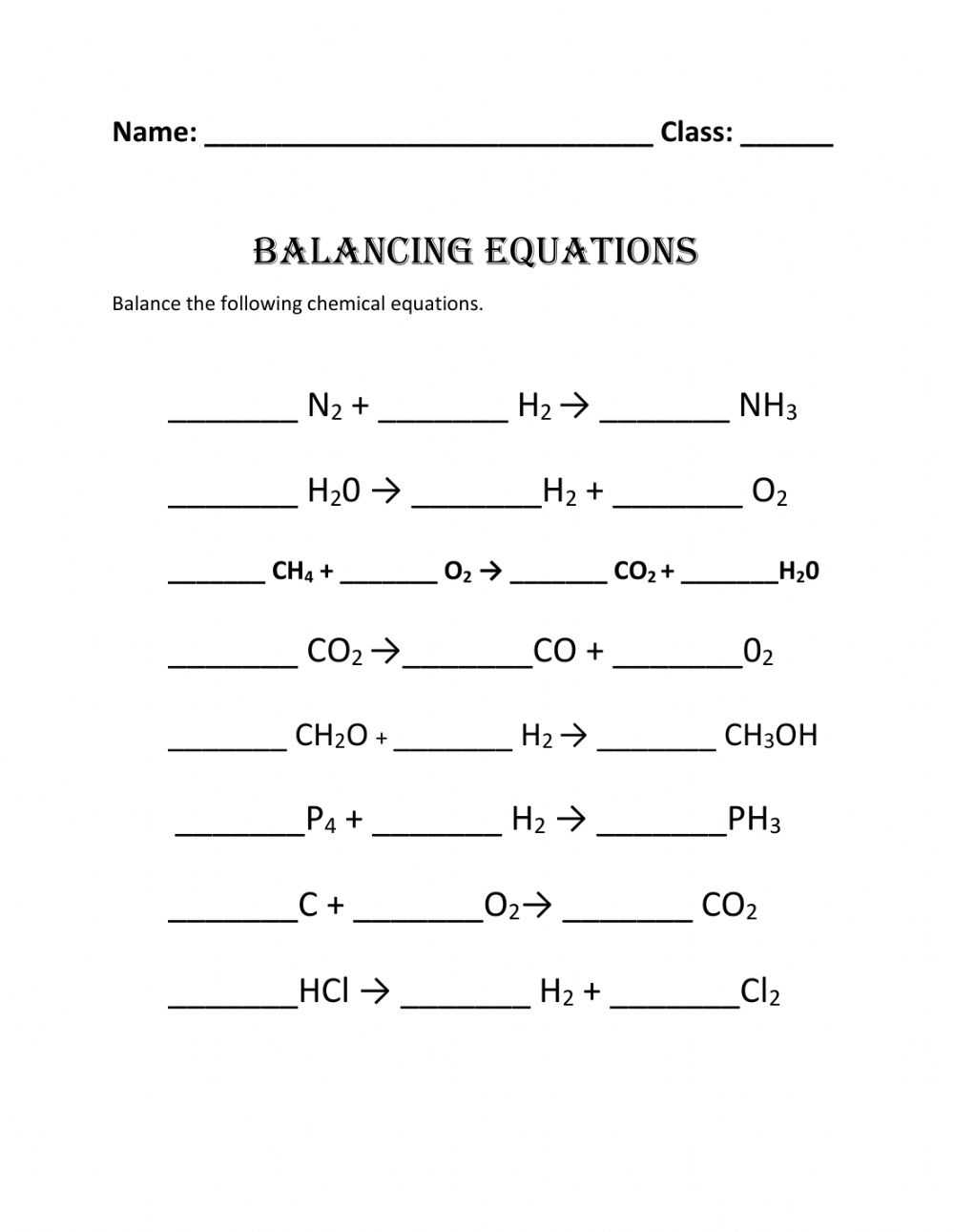
What Is the Purpose of Balancing Chemical Equations Worksheets?
The purpose of balancing chemical equations worksheets is to teach students the fundamentals of chemical equations, which are fundamental to the study of chemistry. Chemical equations are written representations of chemical reactions, which involve the transformation of one or more substances into one or more new substances. This transformation is not always a simple process and requires a precise understanding of the underlying principles and laws of chemistry. Balancing chemical equations worksheets provide students with practice in writing the equations correctly and in understanding their implications. By repeatedly writing and solving equations, students can come to a better understanding of the underlying principles of chemistry and can apply them to future problems.
How to Balance Chemical Equations Using Worksheets
Balancing chemical equations is an essential part of chemistry, as it helps to ensure that the amount of reactants and products in a reaction remains constant. Fortunately, students can make use of worksheets to help them gain a better understanding of the concepts behind the equation and to practice their skills.
To begin, students should find a worksheet that shows the equation in its unbalanced form. They should then identify the reactants and products of the equation, as well as the number of atoms for each element. Next, students should use their knowledge of the law of conservation of mass to determine the amount of atoms that need to be added to each side of the equation in order to balance it. For example, if the equation has two oxygen atoms on one side and three on the other, then one more oxygen atom should be added to the side with two.
[toc]
After the equation is balanced, students should use their knowledge of the coefficients of the equation to determine the correct ratio of reactants and products. The coefficients of the equation indicate the number of molecules of each reactant and product that are needed in order to produce a balanced equation.
Finally, students should check their work by counting the number of atoms on each side of the equation and making sure that they are equal. Once the equation is balanced, students should be able to successfully complete their worksheet and move on to the next step in their chemistry lesson.
Common Mistakes to Avoid When Balancing Chemical Equations Using Worksheets
When balancing chemical equations using worksheets, it is important to take the time to review and understand the equations before attempting to balance them. Here are some common mistakes to avoid:
1. Not accounting for all atoms: Not accounting for all of the atoms in the equation is a common mistake made when balancing equations. Before attempting to balance the equation, count the number of each type of atom on both sides of the equation.
2. Incorrect coefficients: When balancing equations, it is important to ensure that the coefficients are correct. This means that the ratio of the atoms in the equation is correct.
3. Not using the correct symbols: It is important to use the correct symbols when balancing equations. Make sure to check the symbols carefully and if necessary, check a periodic table.
4. Double-checking: Finally, it is important to double-check the equations. Ensure that the coefficients are balanced and that the equation is balanced overall. This is a crucial step in ensuring accuracy.
Conclusion
The Balancing Chemical Equations Worksheet 1 is an excellent way to learn how to properly balance a chemical equation. It provides a step-by-step approach to balancing equations, as well as helpful tips and hints to help students understand the process. By using this worksheet, students can gain a better understanding of balancing chemical equations and be better equipped to tackle more complex chemical equations in the future.
[addtoany]